Asia Pacific Medical Device Regulatory Affairs Outsourcing Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
8.31 Billion
USD
21.78 Billion
2025
2033
USD
8.31 Billion
USD
21.78 Billion
2025
2033
| 2026 –2033 | |
| USD 8.31 Billion | |
| USD 21.78 Billion | |
|
|
|
|
Segmentación del mercado de externalización de asuntos regulatorios de dispositivos médicos en Asia-Pacífico, por servicios (servicios de asuntos regulatorios, consultoría de calidad y redacción médica), producto (productos terminados, electrónica y materia prima), tipo de dispositivo (clase I, clase II y clase III), aplicación (cardiología, diagnóstico por imagen, ortopedia, IVD, oftalmología, cirugía general y plástica, administración de fármacos, odontología, endoscopia, atención diabética y otros), usuario final (pequeña, mediana y gran empresa de dispositivos médicos) - Tendencias del sector y pronóstico hasta 2033
Tamaño del mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
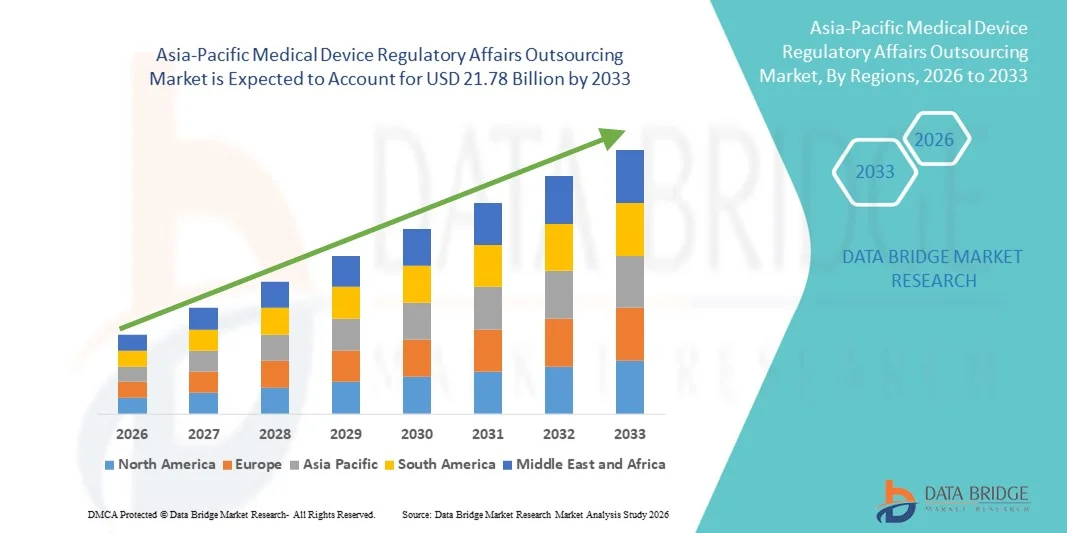
- El tamaño del mercado de subcontratación de asuntos regulatorios de dispositivos médicos de Asia-Pacífico se valoró en USD 8.31 mil millones en 2025 y se espera que alcance los USD 21.78 mil millones para 2033 , con una CAGR del 12,80% durante el período de pronóstico.
- El crecimiento del mercado se ve impulsado en gran medida por la creciente complejidad de los requisitos regulatorios en las industrias farmacéutica, biotecnológica y de dispositivos médicos, lo que impulsa a las empresas a buscar servicios de subcontratación especializados para la gestión del cumplimiento.
- Además, la creciente demanda de presentaciones regulatorias rentables, eficientes y oportunas está impulsando la adopción de soluciones de externalización de asuntos regulatorios. Estos factores convergentes están acelerando la adopción de servicios de externalización de asuntos regulatorios, impulsando así significativamente el crecimiento del sector a nivel mundial.
Análisis del mercado de externalización de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
- El mercado de subcontratación de asuntos regulatorios de dispositivos médicos, que implica delegar la estrategia regulatoria, la documentación de cumplimiento, el registro de productos y las actividades de vigilancia posterior a la comercialización a proveedores de servicios especializados, se está volviendo cada vez más importante para las empresas de dispositivos médicos que operan bajo marcos regulatorios complejos y en evolución en todo el Medio Oriente.
- El creciente rigor de las regulaciones sobre dispositivos médicos, la creciente demanda de aprobaciones más rápidas y la necesidad de experiencia regulatoria local son factores clave que impulsan la adopción de servicios de externalización de asuntos regulatorios de dispositivos médicos. Las empresas recurren a socios externos para minimizar los riesgos de cumplimiento, optimizar los plazos de aprobación y centrarse en las actividades principales de desarrollo y comercialización de productos.
- China dominó el mercado de externalización de asuntos regulatorios de dispositivos médicos, con la mayor participación en los ingresos, aproximadamente el 43,2 % en 2025. Esto se debió a un ecosistema consolidado de atención médica y dispositivos médicos, una sólida supervisión regulatoria por parte de la Administración Nacional de Productos Médicos (NMPA), el aumento de las aprobaciones de dispositivos médicos y la presencia de consultoras regulatorias líderes. El enfoque del país en el cumplimiento normativo, las aprobaciones oportunas y los sólidos procesos de autorización de comercialización han impulsado significativamente la demanda de servicios de externalización especializados.
- Se prevé que India sea el mercado de mayor crecimiento, con una tasa de crecimiento anual compuesta (TCAC) de alrededor del 11,3 % durante el período de pronóstico, impulsada por la rápida modernización de la atención médica, el aumento de las importaciones de dispositivos médicos, la expansión de los servicios de consultoría regulatoria y la evolución de los marcos regulatorios bajo la Organización Central de Control de Estándares de Medicamentos (CDSCO). La creciente concienciación sobre las soluciones eficientes de externalización y el aumento de las inversiones en I+D de dispositivos médicos impulsan aún más la expansión del mercado en todo el país.
- El segmento de productos terminados tuvo la mayor participación en los ingresos del mercado, con un 52,1 %, en 2025, ya que los dispositivos médicos finales están sujetos a rigurosas aprobaciones previas a la comercialización y requisitos de vigilancia posterior a la comercialización.
Alcance del informe y segmentación del mercado de subcontratación de asuntos regulatorios de dispositivos médicos
|
Atributos |
Subcontratación de asuntos regulatorios de dispositivos médicos: Perspectivas clave del mercado |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Asia-Pacífico
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado seleccionados por Data Bridge Market Research también incluyen un análisis en profundidad de expertos, epidemiología de pacientes, análisis de la cartera de productos, análisis de precios y marco regulatorio. |
Tendencias del mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
Aumento de la complejidad de las regulaciones sobre dispositivos médicos en las distintas regiones
- Una tendencia significativa y en auge en el mercado de la externalización de asuntos regulatorios de dispositivos médicos es la creciente complejidad y la continua evolución de los marcos regulatorios que rigen los dispositivos médicos. Los países de la región están reforzando los procesos de aprobación, los requisitos de vigilancia poscomercialización y los estándares de cumplimiento para alinearse mejor con los estándares internacionales.
- Por ejemplo, las autoridades regulatorias de los países del Consejo de Cooperación del Golfo (CCG) y Sudáfrica han introducido sistemas de registro de dispositivos médicos más estructurados, lo que impulsa a los fabricantes a confiar en socios de subcontratación de asuntos regulatorios especializados para navegar eficientemente por los procedimientos de presentación locales y los requisitos de documentación.
- La creciente adopción de normas internacionales, como la ISO 13485 y los sistemas de clasificación basados en riesgos, impulsa la demanda de expertos externos para gestionar la documentación regulatoria, la documentación técnica y las actividades de cumplimiento de calidad. Los socios de externalización proporcionan conocimiento específico para cada región, lo que ayuda a reducir los plazos de aprobación y los riesgos regulatorios.
- Además, la expansión de las empresas multinacionales de dispositivos médicos en los mercados de Oriente Medio y África está fomentando el uso de servicios regulatorios subcontratados para gestionar los registros en varios países a través de un enfoque centralizado y rentable.
- Esta tendencia hacia servicios profesionales de apoyo regulatorio está transformando la manera en que los fabricantes abordan el ingreso al mercado y el cumplimiento, y la subcontratación se está convirtiendo en una necesidad estratégica en lugar de un servicio opcional en toda la región.
- A medida que aumenta el escrutinio regulatorio, la demanda de proveedores de servicios de asuntos regulatorios especializados con experiencia regional continúa creciendo entre los fabricantes de dispositivos médicos tanto globales como locales.
Dinámica del mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
Conductor
Creciente expansión del mercado de dispositivos médicos y rigor regulatorio
- La rápida expansión del sector de dispositivos médicos en todo el mundo, impulsada por el aumento de las inversiones en atención médica, el crecimiento de la población y la creciente prevalencia de enfermedades crónicas, es un impulsor importante para los servicios de subcontratación de asuntos regulatorios.
- Por ejemplo, el creciente desarrollo de la infraestructura de atención de la salud en países como Arabia Saudita, los Emiratos Árabes Unidos y Sudáfrica ha generado una mayor demanda de dispositivos médicos, lo que aumenta la carga de trabajo regulatoria para los fabricantes que buscan aprobaciones de mercado oportunas.
- A medida que las autoridades reguladoras introducen requisitos de cumplimiento más estrictos, los fabricantes subcontratan cada vez más las actividades regulatorias a empresas especializadas para garantizar precisión, consistencia y aprobaciones de productos más rápidas.
- Además, las empresas de dispositivos médicos pequeñas y medianas a menudo carecen de experiencia regulatoria interna para diversas regulaciones regionales, lo que hace que la subcontratación sea una solución práctica para administrar los costos y reducir los riesgos de incumplimiento.
- La necesidad de mantener el cumplimiento normativo durante todo el ciclo de vida del producto, incluidas las renovaciones, las variaciones y la vigilancia posterior a la comercialización, sigue respaldando una demanda sostenida de servicios de subcontratación en la región.
Restricción/Desafío
Armonización regulatoria limitada y escasez de profesionales calificados
- Un desafío clave en el mercado de subcontratación de asuntos regulatorios de dispositivos médicos es la falta de marcos regulatorios armonizados entre los países, lo que aumenta la complejidad y los requisitos de recursos tanto para los proveedores de servicios como para los fabricantes.
- Por ejemplo, la variedad de formatos de presentación, plazos de aprobación y expectativas regulatorias en los mercados de África y Medio Oriente pueden generar demoras y mayores costos operativos, incluso cuando se externalizan las actividades regulatorias.
- Otra limitación importante es la disponibilidad limitada de profesionales regulatorios altamente capacitados con un profundo conocimiento tanto de las regulaciones locales como de los estándares internacionales, particularmente en los mercados africanos emergentes.
- Además, las preocupaciones relacionadas con la confidencialidad de los datos, las brechas de comunicación y la dependencia de proveedores de servicios externos pueden hacer que algunos fabricantes duden antes de externalizar por completo las funciones regulatorias.
- Superar estos desafíos mediante el desarrollo de capacidad regulatoria, iniciativas de armonización regional e inversión en el desarrollo de fuerza laboral calificada será fundamental para el crecimiento y la eficacia a largo plazo del mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Medio Oriente y África.
Alcance del mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
El mercado está segmentado en función de los servicios, el producto, el tipo de dispositivo, la aplicación y el usuario final.
- Por Servicios
En cuanto a los servicios, el mercado de externalización de asuntos regulatorios de dispositivos médicos se segmenta en Servicios de Asuntos Regulatorios, Consultoría de Calidad y Redacción Médica. El segmento de Servicios de Asuntos Regulatorios dominó la mayor cuota de mercado en ingresos, con un 46,8% en 2025, debido a la creciente complejidad de las regulaciones de dispositivos médicos en las principales regiones, como Norteamérica, Europa y Asia-Pacífico. Las empresas recurren a servicios regulatorios externalizados para las presentaciones previas a la comercialización, la documentación técnica y la gestión del cumplimiento normativo posterior a la comercialización. Los crecientes requisitos regulatorios para dispositivos de Clase II y Clase III impulsan la demanda. La globalización de las operaciones de dispositivos médicos y los registros transfronterizos de productos también impulsan este segmento. La externalización de asuntos regulatorios reduce los costes operativos y garantiza el cumplimiento de las directrices de la FDA, el MDR de la UE y el IVDR. El segmento se beneficia del aumento de las auditorías regulatorias y las frecuentes actualizaciones de los estándares de documentación. La gestión del ciclo de vida de los dispositivos, los informes de vigilancia y la mitigación de riesgos son factores clave. Las empresas priorizan cada vez más la externalización para acelerar la comercialización y reducir la carga de recursos internos. La creciente adopción de dispositivos médicos avanzados y la rápida innovación de productos refuerzan aún más su dominio. La expansión hacia los mercados emergentes crea una demanda sostenida de servicios de asuntos regulatorios.
Se espera que el segmento de Redacción Médica experimente la CAGR más rápida del 11,4% entre 2026 y 2033, impulsada por la creciente demanda mundial de informes de evaluación clínica, archivos técnicos y documentación regulatoria de alta calidad. Las pequeñas y medianas empresas de dispositivos médicos dependen cada vez más de redactores médicos externalizados. Las autoridades reguladoras exigen documentación precisa y conforme para la aprobación de dispositivos, lo que impulsa el crecimiento. La adopción de plataformas de presentación electrónica acelera la externalización. El crecimiento de los ensayos clínicos, la recopilación de evidencia del mundo real y los informes de datos posteriores a la comercialización aumenta la demanda. La digitalización y las herramientas de redacción médica asistidas por IA contribuyen a una preparación más rápida de documentos. Las empresas buscan contratos de externalización flexibles para reducir la carga de trabajo interna. La expansión de áreas terapéuticas, como cardiología, ortopedia y dispositivos de diagnóstico in vitro (IVD), impulsa aún más el crecimiento. La creciente armonización regulatoria internacional respalda las necesidades de externalización transfronteriza. En general, el segmento se beneficia de la creciente complejidad regulatoria y la creciente importancia de una documentación médica precisa.
- Por producto
En función del producto, el mercado de externalización de asuntos regulatorios de dispositivos médicos se segmenta en productos terminados, electrónica y materias primas. El segmento de productos terminados registró la mayor cuota de mercado, con un 52,1 % en 2025, ya que los dispositivos médicos finales están sujetos a rigurosas aprobaciones previas a la comercialización y requisitos de vigilancia poscomercialización. Las empresas externalizan los procesos regulatorios para garantizar el cumplimiento sin problemas de la FDA, el MDR de la UE y otras normativas regionales. Los productos terminados incluyen dispositivos para cardiología, ortopedia, diagnóstico in vitro (IVD) y oftalmología. La externalización ayuda a reducir la carga de trabajo interna, a la vez que garantiza el cumplimiento de los estándares de calidad y seguridad. Los fabricantes buscan apoyo experto para el etiquetado, la documentación, la validación y la preparación de evidencia clínica. Un mayor escrutinio de los productos combinados, los dispositivos integrados en software y los dispositivos médicos conectados refuerza su dominio del mercado. La expansión global de las operaciones de dispositivos médicos impulsa los contratos de externalización a largo plazo. La externalización mitiga los riesgos asociados a las auditorías y las sanciones por incumplimiento. Las empresas priorizan la rapidez de comercialización sin comprometer el cumplimiento. La vigilancia poscomercialización y la notificación de eventos adversos son factores adicionales. Los fabricantes buscan optimizar la asignación de recursos y la rentabilidad mediante la subcontratación.
Se anticipa que el segmento de Electrónica experimentará la CAGR más rápida del 10.7% de 2026 a 2033, debido a la creciente integración de software y componentes digitales en dispositivos médicos. El cumplimiento normativo para software, firmware y ciberseguridad de dispositivos conectados es complejo y está en constante evolución. Se prefiere la subcontratación para garantizar la preparación regulatoria y aprobaciones más rápidas. El crecimiento en dispositivos portátiles, sistemas de monitoreo remoto y soluciones de salud digital respalda el segmento. Las empresas emergentes y las pymes recurren cada vez más a socios reguladores expertos para dispositivos con gran cantidad de electrónica. La adopción de IA y aprendizaje automático en dispositivos médicos aumenta los requisitos de documentación y pruebas. La comercialización transfronteriza impulsa la demanda de documentación regulatoria estandarizada. La tendencia de las soluciones de salud conectada acelera la subcontratación de las actividades de cumplimiento. Las empresas buscan reducir la carga operativa interna mientras se adhieren a los estándares regulatorios globales. La expansión de los dispositivos de telemedicina y los dispositivos médicos habilitados para IoT respalda aún más el crecimiento.
- Por tipo de dispositivo
Según el tipo de dispositivo, el mercado de externalización de asuntos regulatorios de dispositivos médicos se segmenta en dispositivos de Clase I, Clase II y Clase III. El segmento de dispositivos de Clase II dominó el mercado con una participación en los ingresos del 41,6 % en 2025, debido al alto volumen de dispositivos moderadamente regulados que ingresaron al mercado. Las presentaciones regulatorias para dispositivos de Clase II implican documentación, archivos técnicos y el cumplimiento de las directrices 510(k) de la FDA o del marcado CE. La externalización ayuda a los fabricantes a agilizar las aprobaciones y reducir la carga de trabajo interna. Los dispositivos ortopédicos, de diagnóstico y de monitorización constituyen una parte importante de los productos de Clase II. Las empresas aprovechan la experiencia externalizada para gestionar el ciclo de vida del producto, la vigilancia poscomercialización y las auditorías regulatorias. La globalización de las operaciones de dispositivos y el aumento de la innovación impulsan aún más la demanda. La reducción de costos, la eficiencia y la entrada oportuna al mercado son ventajas clave. La complejidad regulatoria en múltiples regiones refuerza la dependencia de la externalización. Las actividades de gestión del ciclo de vida, que incluyen modificaciones, actualizaciones e informes de dispositivos, impulsan el segmento. La creciente adopción de dispositivos médicos en los mercados emergentes contribuye a su dominio. Los dispositivos de Clase II representan un equilibrio entre innovación y complejidad regulatoria, lo que hace que la subcontratación sea esencial.
Se proyecta que el segmento de dispositivos de Clase III crecerá a la tasa de crecimiento anual compuesta (TCAC) más rápida del 12,8 % entre 2026 y 2033, impulsada por el creciente desarrollo de dispositivos implantables, de soporte vital y de alto riesgo. Estos dispositivos requieren amplia evidencia clínica, estudios de validación y aprobaciones regulatorias. La externalización de los asuntos regulatorios minimiza los retrasos en las aprobaciones y garantiza el cumplimiento de las normas globales. El escrutinio de dispositivos de alto riesgo, incluidos los implantes cardíacos y los dispositivos de neuromodulación, impulsa el crecimiento del mercado. Los fabricantes dependen de socios regulatorios para la documentación, los informes de evaluación clínica y los archivos técnicos. La creciente tendencia de productos combinados aumenta la demanda de externalización. La vigilancia continua posterior a la comercialización y los requisitos de notificación de eventos adversos respaldan aún más la expansión. Las actualizaciones regulatorias bajo MDR e IVDR impulsan el crecimiento del segmento. Las pequeñas y medianas empresas externalizan cada vez más el cumplimiento de los dispositivos de Clase III debido a la complejidad. La innovación en materiales biocompatibles e implantes inteligentes impulsa la aceleración del segmento. Los productos de alto valor del segmento y el riesgo regulatorio impulsan la demanda sostenida de externalización.
- Por aplicación
Según la aplicación, el mercado de externalización de asuntos regulatorios de dispositivos médicos se segmenta en cardiología, diagnóstico por imagen, ortopedia, IVD, oftalmología, cirugía general y plástica, administración de fármacos, odontología, endoscopia, atención diabética y otros. El segmento IVD representó la mayor cuota de mercado en ingresos, con un 30,2%, en 2025, impulsado por la creciente demanda de pruebas diagnósticas y medicina personalizada. La externalización es crucial debido a los estrictos requisitos de cumplimiento de la UE IVDR y la FDA. Los fabricantes dependen de expertos en regulación para la aprobación, validación, etiquetado y seguimiento poscomercialización de dispositivos. La expansión de centros de diagnóstico, laboratorios clínicos e instalaciones de diagnóstico molecular impulsa el crecimiento. El aumento de la prevalencia de enfermedades crónicas impulsa la demanda de dispositivos de diagnóstico precisos. Las empresas buscan reducir los costes operativos y mitigar los riesgos regulatorios. La globalización de la distribución de dispositivos IVD refuerza aún más los requisitos de externalización. La rápida innovación de productos requiere apoyo regulatorio oportuno. La clasificación basada en riesgos de los IVD impulsa la dependencia de la externalización. Las auditorías regulatorias, el marcado CE y las autorizaciones de la FDA aumentan la actividad del mercado. El segmento se beneficia de las crecientes inversiones en detección temprana de enfermedades y medicina de precisión.
Se espera que el segmento de Cuidado de la Diabetes experimente la CAGR más rápida del 13.3% de 2026 a 2033, impulsada por el aumento global en la prevalencia de la diabetes y la adopción de sistemas conectados de monitoreo de glucosa. Los requisitos regulatorios para monitores continuos de glucosa y dispositivos inteligentes de administración de insulina son complejos, lo que aumenta la demanda de subcontratación. Las empresas emergentes y los fabricantes de tamaño mediano dependen de socios expertos para la documentación, los archivos técnicos y el cumplimiento. Los dispositivos de control de la diabetes integrados con IA requieren soporte regulatorio adicional. El crecimiento de los dispositivos portátiles y las soluciones de cuidado en el hogar respalda la expansión del segmento. La entrada al mercado transfronterizo exige presentaciones regulatorias armonizadas. La integración de la terapéutica digital y la telemedicina impulsa aún más la subcontratación. La vigilancia posterior a la comercialización y la gestión de riesgos son factores críticos. El enfoque de los gobiernos y los proveedores de atención médica en el control de la diabetes fortalece el mercado. El aumento de la financiación de riesgo en soluciones digitales para la diabetes acelera el crecimiento. Los fabricantes a pequeña escala se benefician de la reducción de los costos de cumplimiento interno. La brecha de conocimiento regulatorio entre las nuevas empresas promueve una alta adopción de la subcontratación.
- Por el usuario final
En función del usuario final, el mercado de externalización de asuntos regulatorios de dispositivos médicos se segmenta en pequeñas empresas de dispositivos médicos, medianas empresas de dispositivos médicos y grandes empresas de dispositivos médicos. El segmento de grandes empresas de dispositivos médicos dominó el mercado con una participación en los ingresos del 44,9 % en 2025, gracias a su amplia cartera de productos y operaciones globales. La externalización facilita la presentación de solicitudes regulatorias en múltiples países y garantiza el cumplimiento de diversas normas. Los frecuentes lanzamientos de productos y las actividades de gestión del ciclo de vida requieren el apoyo de expertos. Las empresas aprovechan la externalización para optimizar la asignación de recursos y reducir la carga de cumplimiento interno. Las grandes empresas se enfrentan a un alto escrutinio regulatorio para los dispositivos de Clase II y III, lo que impulsa el crecimiento del segmento. La vigilancia poscomercialización, las evaluaciones clínicas y la documentación técnica se externalizan a empresas especializadas. Las auditorías regulatorias, el marcado CE y las aprobaciones 510(k) de la FDA impulsan la adopción de la externalización. La mitigación de riesgos, la rentabilidad y una entrada más rápida al mercado impulsan el dominio. La expansión a mercados emergentes requiere experiencia en externalización. La integración de software y dispositivos conectados impulsa aún más la demanda. La externalización permite a las grandes empresas centrarse en la I+D, garantizando al mismo tiempo el cumplimiento normativo.
Se prevé que el segmento de pequeñas empresas de dispositivos médicos crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida del 13,1 % entre 2026 y 2033, impulsada por la limitada experiencia regulatoria interna. Las empresas emergentes y las empresas en fase inicial dependen en gran medida de servicios externalizados para cumplir con los requisitos de cumplimiento global. La externalización ayuda a reducir los plazos de aprobación y los costes operativos. La creciente innovación en dispositivos portátiles, de diagnóstico y de atención domiciliaria impulsa el crecimiento. La documentación compleja, la evaluación clínica y los requisitos posteriores a la comercialización hacen que la externalización sea esencial. Las empresas financiadas con capital de riesgo buscan soluciones regulatorias rentables. La expansión del mercado global exige experiencia en regulaciones regionales. La rápida evolución de los requisitos de MDR e IVDR acelera la adopción de la externalización. Las herramientas regulatorias asistidas por IA ayudan a las pequeñas empresas a lograr una documentación eficiente. La mitigación de riesgos de cumplimiento es un importante impulsor del crecimiento del segmento. Las empresas emergentes aprovechan la externalización para una comercialización más rápida y una menor carga de trabajo interna. El aumento de las asociaciones con CRO y consultores regulatorios fortalece las perspectivas de crecimiento.
Análisis regional del mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
- Se proyecta que el mercado de subcontratación de asuntos regulatorios de dispositivos médicos de Asia-Pacífico crecerá de manera constante durante el período de pronóstico, respaldado por el fortalecimiento de los marcos regulatorios, el aumento de las aprobaciones de dispositivos médicos y la expansión de la infraestructura de atención médica en toda la región.
- Los gobiernos priorizan cada vez más el cumplimiento normativo, el registro de productos y la vigilancia poscomercialización para garantizar la seguridad del paciente y los estándares de calidad. Como resultado, los fabricantes de dispositivos médicos externalizan cada vez más las actividades de asuntos regulatorios a proveedores de servicios especializados para gestionar eficientemente los complejos y cambiantes requisitos regulatorios.
- Las crecientes inversiones en la modernización de la atención sanitaria, junto con la creciente adopción de tecnologías médicas avanzadas, están acelerando aún más la demanda de servicios de subcontratación de asuntos regulatorios en toda la región de Asia y el Pacífico.
Análisis del mercado de externalización de asuntos regulatorios de dispositivos médicos en China.
El mercado chino de externalización de asuntos regulatorios de dispositivos médicos dominó el mercado de externalización de asuntos regulatorios de dispositivos médicos en Asia-Pacífico, representando aproximadamente el 43,2 % de los ingresos regionales en 2025. Este dominio se debe a un ecosistema consolidado de atención médica y dispositivos médicos, una sólida supervisión regulatoria por parte de la Administración Nacional de Productos Médicos (NMPA), el aumento de las aprobaciones de dispositivos médicos y la presencia de consultoras regulatorias líderes. El enfoque del país en el cumplimiento normativo, las aprobaciones oportunas y los sólidos procesos de autorización de comercialización ha impulsado significativamente la demanda de servicios de externalización especializados en hospitales, fabricantes de dispositivos médicos y CDMO.
Análisis del mercado de externalización de asuntos regulatorios de dispositivos médicos en India.
Se prevé que el mercado de externalización de asuntos regulatorios de dispositivos médicos en India sea el de mayor crecimiento, con una tasa de crecimiento anual compuesta (TCAC) de alrededor del 11,3 % durante el período de pronóstico. Este crecimiento se sustenta en la rápida modernización de la atención médica, el aumento de las importaciones de dispositivos médicos, la expansión de los servicios de consultoría regulatoria y la evolución de los marcos regulatorios bajo la Organización Central de Control de Estándares de Medicamentos (CDSCO). La creciente concienciación sobre las soluciones de externalización eficientes, junto con el aumento de las inversiones en I+D de dispositivos médicos y la afluencia de fabricantes internacionales de dispositivos médicos, impulsan aún más la expansión del mercado en todo el país.
Cuota de mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
La industria de subcontratación de asuntos regulatorios de dispositivos médicos está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Accell Clinical Research, LLC (EE. UU.)
- Genpact (EE. UU.)
- CRITERIUM, INC. (EE. UU.)
- Promedica International (EE. UU.)
- WuXiAppTec (China)
- Medpace (EE. UU.)
- PPD Inc. (EE. UU.)
- Laboratorios Charles River (EE. UU.)
- ICON plc (EE. UU.)
- Covance (EE. UU.)
- Parexel International Corporation (EE. UU.)
- Freyr
- Navitas Clinical Research, Inc. (EE. UU.)
- Medelis, Inc. (EE. UU.)
- Sciformix (EE. UU.)
- Tech Tammina (EE. UU.)
- Acorn Regulatory Consultancy Services Ltd. (Irlanda)
- BIOMAPAS (Lituania)
- PROFESIONALES REGULADORES (Australia)
- CompareNetworks, Inc. (EE. UU.)
Últimos avances en el mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Asia-Pacífico
- En enero de 2023, Medistri SA, proveedor suizo de servicios de tecnología médica, presentó una solución integrada de consultoría en asuntos regulatorios y gestión de calidad, diseñada para pequeños y medianos fabricantes de dispositivos médicos. Esta solución simplifica el cumplimiento normativo al brindar soporte rentable para el marcado CE, la preparación de expedientes técnicos y la vigilancia poscomercialización, satisfaciendo así una necesidad clave para las empresas que carecen de equipos regulatorios internos completos.
- En marzo de 2023, ICON plc lanzó una nueva plataforma de Inteligencia Regulatoria diseñada para ayudar a las empresas de dispositivos médicos a monitorear la evolución de las regulaciones globales, gestionar las estrategias de presentación y acceder a plantillas de cumplimiento. La plataforma tenía como objetivo mejorar la estrategia regulatoria y la eficiencia operativa para presentaciones complejas en múltiples jurisdicciones.
- En marzo de 2023, Freyr Solutions inauguró un nuevo centro global de servicios regulatorios en la región Asia-Pacífico, ampliando su capacidad para apoyar a las empresas de dispositivos médicos en el cumplimiento integral de las aprobaciones específicas de cada país. Esta oferta regional mejorada de servicios puso de relieve el rápido crecimiento de la demanda de externalización regulatoria en los mercados emergentes.
- En abril de 2023, Parexel International Corporation amplió sus servicios globales de consultoría regulatoria para brindar un mejor apoyo a los desarrolladores de dispositivos médicos y productos combinados que se enfrentan a los requisitos cada vez más complejos del Reglamento de Dispositivos Médicos (MDR) y el Reglamento de Diagnóstico In Vitro (IVDR) de la UE. Esta mejora estratégica reflejó la creciente demanda del sector de conocimientos especializados en MDR/IVDR.
- En marzo de 2024, Emergo by UL estableció un nuevo centro de consultoría regulatoria en Singapur, enfocado en atender el creciente mercado de dispositivos médicos de Asia-Pacífico, en particular apoyando las iniciativas de armonización regulatoria de la ASEAN y las aprobaciones multinacionales. Esta iniciativa reforzó la sólida expansión geográfica de los servicios regulatorios externalizados.
- En julio de 2024, Parexel International lanzó su Plataforma Regulatoria Digital, que integra inteligencia artificial y aprendizaje automático para agilizar las presentaciones regulatorias de dispositivos médicos, proporcionar gestión de documentos basada en la nube y mejorar la colaboración entre patrocinadores y reguladores.
- En septiembre de 2024, IQVIA amplió sus capacidades de asuntos regulatorios mediante la adquisición de la división de consultoría regulatoria de Pharm‑Olam, reforzando su experiencia regulatoria global en dispositivos médicos y mejorando los servicios en Asia-Pacífico y América Latina, especialmente para las estrategias regulatorias de mercados emergentes.
- En enero de 2025, ProPharma Group se asoció con MedTech Europe para desarrollar programas de capacitación regulatoria especializados para profesionales de la regulación de dispositivos médicos, abordando brechas críticas de habilidades luego de la implementación de marcos regulatorios más estrictos como MDR.
- En febrero de 2025, IQVIA anunció una asociación estratégica con un fabricante europeo de dispositivos médicos para respaldar la preparación de expedientes regulatorios y los informes de evaluación clínica según el MDR de la UE, lo que refuerza la dependencia de la industria en la experiencia subcontratada para la documentación de cumplimiento compleja.
- En marzo de 2025, los análisis del mercado indicaron que ICON plc expandió sus servicios de subcontratación de asuntos regulatorios en la región Asia-Pacífico, respondiendo a la creciente demanda localizada de consultoría regulatoria y apoyo para ensayos clínicos en mercados de alto crecimiento como China e India.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.