Asia Pacific Anti Nuclear Antibody Test Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
558.77 Million
USD
1,639.25 Million
2024
2032
USD
558.77 Million
USD
1,639.25 Million
2024
2032
| 2025 –2032 | |
| USD 558.77 Million | |
| USD 1,639.25 Million | |
|
|
|
|
Segmentación del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico, por tipo de anticuerpo (antígenos nucleares extraíbles [ENA], anti-DSDNA e histonas, anticuerpos anti-DFS70, anti-PM-SCL, anticuerpos anticentrómero, anti-SP100 y otros), producto (instrumentos, consumibles y reactivos, y servicios), técnica (ELISA, inmunofluorescencia indirecta [IFI], prueba de transferencia, microarray de antígenos, técnicas basadas en gel, ensayo multiplex, citometría de flujo, hemaglutinación pasiva [PHA] y otras), aplicación (enfermedades autoinmunes e infecciosas), por usuario final (hospitales, laboratorios, centros de diagnóstico, institutos de investigación y otros), canal de distribución (licitación directa, ventas minoristas, distribuidores externos y otros) - Tendencias del sector y pronóstico hasta 2032
Tamaño del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
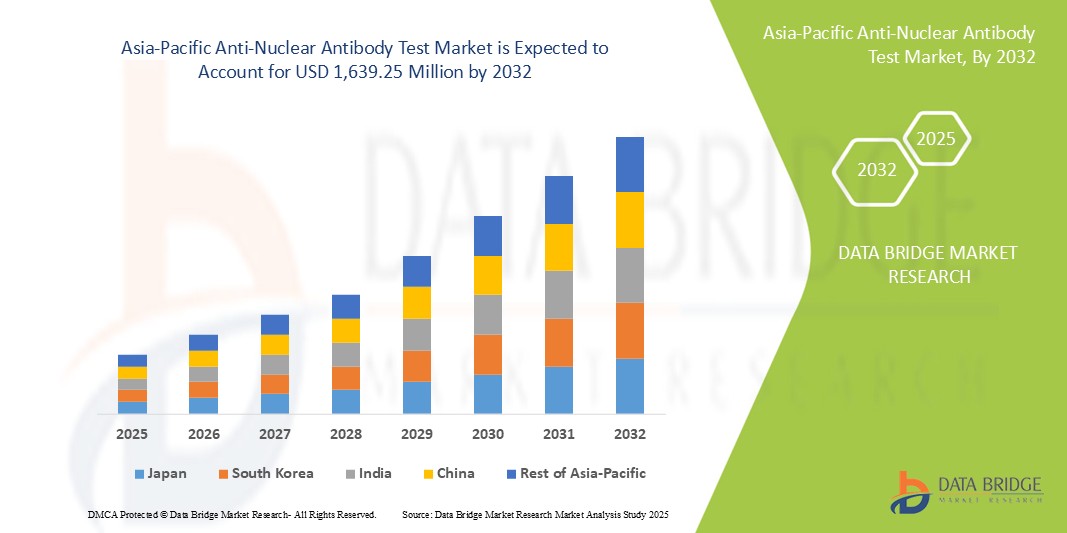
- El tamaño del mercado de pruebas de anticuerpos antinucleares de Asia-Pacífico se valoró en USD 558,77 millones en 2024 y se espera que alcance los USD 1.639,25 millones para 2032 , con una CAGR del 14,40 % durante el período de pronóstico.
- Este crecimiento está impulsado por factores como la creciente prevalencia de enfermedades autoinmunes, los avances tecnológicos en el diagnóstico y la creciente concienciación sobre la atención sanitaria.
Análisis del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
- Las pruebas de anticuerpos antinucleares (ANA) son herramientas diagnósticas esenciales para detectar autoanticuerpos en sangre, lo que facilita el diagnóstico de enfermedades autoinmunes como el lupus eritematoso sistémico (LES), la artritis reumatoide y el síndrome de Sjögren. Estas pruebas desempeñan un papel fundamental en la detección temprana y el tratamiento de estas afecciones.
- La creciente incidencia de trastornos autoinmunes, como el lupus eritematoso sistémico (LES) y la artritis reumatoide, está impulsando la demanda de pruebas de ANA en toda la región.
- Se espera que China domine el mercado de pruebas de anticuerpos antinucleares de Asia-Pacífico con una participación de mercado del 32,4%, impulsada por su gran base poblacional, la creciente prevalencia de enfermedades autoinmunes y las importantes inversiones en infraestructura de atención médica.
- Se espera que India sea el país de más rápido crecimiento con una CAGR del 14,6 % en el mercado de pruebas de anticuerpos antinucleares de Asia-Pacífico, impulsado por la creciente concienciación sobre la atención médica y el aumento del gasto en atención médica.
- Se espera que ELISA (ensayo inmunoabsorbente ligado a enzimas) domine el mercado con una participación de mercado del 52,60% debido a su alta sensibilidad, rentabilidad y amplia disponibilidad, lo que lo convierte en la opción preferida para la detección a gran escala en laboratorios clínicos.
Alcance del informe y segmentación del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
|
Atributos |
Análisis clave del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Asia-Pacífico
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis de importación y exportación, descripción general de la capacidad de producción, análisis del consumo de producción, análisis de tendencias de precios, escenario de cambio climático, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
Avances en las pruebas y automatización de anticuerpos antinucleares (ANA) para el diagnóstico autoinmunitario en Asia-Pacífico
- Una tendencia notable en la evolución de las pruebas de ANA en la región de Asia y el Pacífico es el uso creciente de plataformas de pruebas automatizadas y tecnologías de diagnóstico mejoradas.
- Estas innovaciones mejoran significativamente la velocidad, precisión y eficiencia de las pruebas de ANA, proporcionando mayor sensibilidad y confiabilidad en la detección de trastornos autoinmunes.
- Por ejemplo, los sistemas automatizados de ensayo de inmunofluorescencia (IFA) están agilizando el proceso de prueba, lo que permite a los laboratorios manejar mayores volúmenes de muestras con menos errores humanos y tiempos de respuesta más rápidos, lo cual es crucial para un diagnóstico preciso de enfermedades autoinmunes.
- El auge en el desarrollo y la adopción de dispositivos de prueba en el punto de atención contribuye aún más al crecimiento del mercado. Estos dispositivos proporcionan resultados más rápidos, lo que facilita la toma de decisiones terapéuticas oportunas, especialmente en zonas remotas con acceso limitado a atención médica especializada.
- Estos avances están transformando el diagnóstico autoinmune en Asia-Pacífico, mejorando la precisión del diagnóstico y optimizando la detección temprana de enfermedades autoinmunes, impulsando así la demanda de soluciones de prueba de ANA más eficientes y accesibles.
Dinámica del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
Conductor
“Creciente prevalencia de enfermedades autoinmunes”
- La creciente prevalencia de enfermedades autoinmunes como el lupus eritematoso sistémico (LES), la artritis reumatoide y los trastornos tiroideos autoinmunes está impulsando significativamente la demanda de pruebas de ANA en la región de Asia y el Pacífico.
- A medida que la población de muchos países de Asia y el Pacífico envejece y los factores ambientales contribuyen a los cambios en el sistema inmunológico, hay un aumento notable en la incidencia de enfermedades autoinmunes, particularmente entre las mujeres.
- A medida que más personas son diagnosticadas con estas afecciones, la demanda de pruebas ANA está aumentando, lo que garantiza un diagnóstico preciso y mejores resultados en el manejo de la enfermedad.
Por ejemplo,
- En octubre de 2023, la Organización Mundial de la Salud (OMS) informó que la prevalencia de enfermedades autoinmunes en Asia está aumentando, especialmente en países como China e India, que tienen grandes poblaciones envejecidas y una creciente conciencia sobre la atención médica.
- Como resultado, la creciente incidencia de enfermedades autoinmunes ha creado una necesidad urgente de pruebas de ANA, expandiendo así el mercado de herramientas de diagnóstico.
Oportunidad
Avances tecnológicos en las pruebas de ANA
- El desarrollo de plataformas de diagnóstico automatizadas de alto rendimiento para las pruebas de ANA ofrece una oportunidad significativa para aumentar la eficiencia y la precisión de las pruebas.
- La automatización puede reducir el riesgo de error humano, aumentar el rendimiento en los laboratorios y acelerar el proceso de diagnóstico, haciéndolo más accesible a poblaciones más grandes, especialmente en regiones rurales o desatendidas.
- Además, la adopción de dispositivos de prueba en el punto de atención (POC) para las pruebas de ANA presenta una oportunidad para mejorar el diagnóstico en lugares con acceso limitado a laboratorios especializados.
Por ejemplo,
- En marzo de 2024, un estudio publicado en el Asian Journal of Clinical Immunology destacó que los sistemas automatizados de IFA (ensayo de inmunofluorescencia) se están volviendo cada vez más populares en países como Japón y Corea del Sur, mejorando la precisión y la eficiencia general de las pruebas en entornos clínicos con mucha actividad.
- Al aprovechar la automatización y la tecnología POC, las pruebas de ANA se pueden realizar más rápidamente, lo que proporciona resultados de diagnóstico más rápidos y permite intervenciones médicas oportunas para enfermedades autoinmunes.
Restricción/Desafío
“Alto costo y acceso limitado a la infraestructura de diagnóstico”
- El costo de las pruebas de ANA, junto con la disponibilidad limitada de equipos de diagnóstico avanzados en áreas rurales o en desarrollo, plantea un desafío para el crecimiento del mercado en la región de Asia y el Pacífico.
- Aunque las pruebas de ANA son cruciales para diagnosticar enfermedades autoinmunes, el gasto de mantener sistemas de diagnóstico avanzados puede ser una barrera para los proveedores de atención médica en entornos de bajos recursos.
- Además, la necesidad de técnicos de laboratorio altamente calificados y la naturaleza compleja de las pruebas de ANA limitan el acceso a estos servicios de diagnóstico en ciertas regiones.
Por ejemplo,
- En agosto de 2023, un artículo publicado por la Asociación Médica de la India enfatizó el desafío de la asequibilidad y la infraestructura, particularmente en las zonas rurales de la India, donde el acceso a servicios de diagnóstico avanzados es limitado debido a restricciones financieras y a la falta de profesionales capacitados.
- Como resultado, los sistemas de atención médica en estas regiones pueden tener dificultades para proporcionar pruebas de ANA oportunas y precisas, lo que obstaculiza el crecimiento general del mercado en la región.
Alcance del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
El mercado está segmentado según el tipo de anticuerpo, producto, técnica, aplicación, usuario final y canal de distribución.
|
Segmentación |
Subsegmentación |
|
Por tipo de anticuerpo |
|
|
Por producto |
|
|
Por técnica |
|
|
Por aplicación
|
|
|
Por el usuario final |
|
|
Por canal de distribución |
|
Se proyecta que en 2025, la prueba ELISA dominará el mercado con la mayor participación en el segmento técnico.
Se prevé que el segmento ELISA (ensayo inmunoabsorbente ligado a enzimas) domine el mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico, con la mayor cuota de mercado, un 52,60 %, en 2025. Este dominio se debe principalmente a su alta sensibilidad, rentabilidad y amplia disponibilidad, lo que lo convierte en la opción preferida para el cribado a gran escala en laboratorios clínicos. La fiabilidad y la eficiencia del ELISA en la detección de enfermedades autoinmunes contribuyen aún más a su liderazgo en el mercado.
Se espera que los antígenos nucleares extraíbles (ENA) representen la mayor participación durante el período de pronóstico en el mercado de tipos de anticuerpos.
En 2025, se prevé que el segmento de antígenos nucleares extraíbles (ENA) domine el mercado con la mayor cuota de mercado, un 29,35 %. Este predominio se debe principalmente al papel crucial de las pruebas de ENA en el diagnóstico de enfermedades autoinmunes específicas, como el lupus eritematoso sistémico (LES) y el síndrome de Sjögren. La precisión y especificidad de las pruebas de ENA las convierten en un componente vital del diagnóstico autoinmune, lo que impulsa su liderazgo en el mercado.
Análisis regional del mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
- China tiene una participación dominante en el mercado de pruebas ANA de Asia-Pacífico con una participación de mercado del 32,4 %, impulsada por su gran base poblacional, la creciente prevalencia de enfermedades autoinmunes y las importantes inversiones en infraestructura de atención médica.
- Japón es un país líder en la región Asia-Pacífico, conocido por sus tecnologías sanitarias avanzadas, su alto gasto sanitario y su fuerte enfoque en la detección temprana de enfermedades. Japón aporta aproximadamente el 20 % del mercado regional de pruebas ANA, lo que refleja su consolidado sistema sanitario y el envejecimiento de su población.
- Se proyecta que India experimente la tasa de crecimiento anual compuesta (CAGR) más alta del mercado, con una participación de mercado del 14,6 %, impulsada por la creciente conciencia sobre la atención médica y el aumento del gasto en atención médica.
- Los países del sudeste asiático, incluidos Indonesia, Malasia y Filipinas, están experimentando un rápido crecimiento del mercado debido al aumento de las inversiones en atención médica, la creciente conciencia de las enfermedades autoinmunes y la expansión de las capacidades de diagnóstico.
- Australia y Nueva Zelanda también están experimentando un crecimiento constante del mercado, respaldado por altos estándares de atención médica, una amplia cobertura de seguros y una creciente población de personas mayores.
- La creciente tendencia de los diagnósticos en el punto de atención (POC) en Asia-Pacífico, especialmente en regiones remotas y rurales, está impulsando la demanda de pruebas de ANA. Soluciones de diagnóstico en el punto de atención en la región.
Prueba de anticuerpos antinucleares de Asia-Pacífico
El panorama competitivo del mercado ofrece detalles por competidor. Se incluye información general de la empresa, sus estados financieros, ingresos generados, potencial de mercado, inversión en investigación y desarrollo, nuevas iniciativas de mercado, presencia global, plantas de producción, capacidad de producción, fortalezas y debilidades de la empresa, lanzamiento de productos, alcance y variedad de productos, y dominio de las aplicaciones. Los datos anteriores se refieren únicamente al enfoque de mercado de las empresas.
Los principales líderes del mercado que operan en el mercado son:
- Thermo Fisher Scientific Inc. (EE. UU.)
- Bio-Rad Laboratories, Inc. (EE. UU.)
- Abbott (EE. UU.)
- Euroimmun Medizinische Labordiagnostika AG (Alemania)
- Revvity Inc. (EE. UU.)
- Trinity Biotech (Irlanda)
- LIFESPAN BIOSCIENCES, INC. (EE. UU.)
- ORIGENE TECHNOLOGIES, INC. (EE. UU.)
- Corporación Abnova (Taiwán)
- CUSABIO TECHNOLOGY LLC (EE. UU.)
- Biorbyt Ltd. (Inglaterra)
Últimos avances en el mercado de pruebas de anticuerpos antinucleares en Asia-Pacífico
- En marzo de 2025, Shenzhen Mindray Bio-Medical Electronics Co., Ltd. anunció el lanzamiento de su plataforma de análisis de ANA de última generación en la región Asia-Pacífico, diseñada para mejorar la precisión y el rendimiento de las pruebas. El nuevo sistema integra automatización avanzada e inteligencia artificial para proporcionar resultados rápidos y fiables para el diagnóstico de enfermedades autoinmunes, lo que facilita la toma de decisiones clínicas.
- En febrero de 2025, Sysmex Corporation, empresa japonesa líder en diagnóstico, presentó su plataforma ELISA mejorada para pruebas de ANA, con mayor sensibilidad y precisión. Este desarrollo busca abordar la creciente demanda de diagnósticos autoinmunes precisos en el mercado de Asia-Pacífico, impulsada por la creciente concienciación sobre las enfermedades autoinmunes y la expansión de la infraestructura sanitaria.
- En enero de 2025, Bio-Rad Laboratories, Inc. amplió su cartera de pruebas de ANA en la región Asia-Pacífico con la introducción de un nuevo sistema de análisis multiplex, que ofrece un perfilado completo de autoanticuerpos para un diagnóstico más preciso de la enfermedad. Este lanzamiento se alinea con la estrategia de la compañía de fortalecer su presencia en el creciente sector de diagnóstico de Asia-Pacífico.
- En diciembre de 2024, MBL (Medical & Biological Laboratories Co., Ltd.) presentó su nueva línea de reactivos de diagnóstico para la detección de ANA en el mercado de Asia-Pacífico. Estos reactivos están diseñados para proporcionar resultados altamente específicos y precisos, lo que facilita el diagnóstico temprano y el manejo eficaz de enfermedades autoinmunes como el lupus eritematoso sistémico (LES) y la artritis reumatoide.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.