Según el informe "Uretritis por Mycoplasma genitalium: Prevalencia y patrones de resistencia a los medicamentos" publicado en marzo de 2020, Mycoplasma gentitalium es una de las principales causas de uretritis masculina, y según un estudio realizado en un total de 914 participantes, el 28,7% de los La población tenía infección por Mycoplasma genitalium (MG). Según las noticias de febrero de 2019, se aisló Mycoplasma del recto y del canal anal de pacientes con enfermedad de Crohn, lo que implica que Mycoplasma también es responsable de infecciones gastrointestinales prevalentes.
Acceda al informe completo enhttps://databridgemarketresearch.com/reports/global-mycoplasma-testing-in-clinical-market
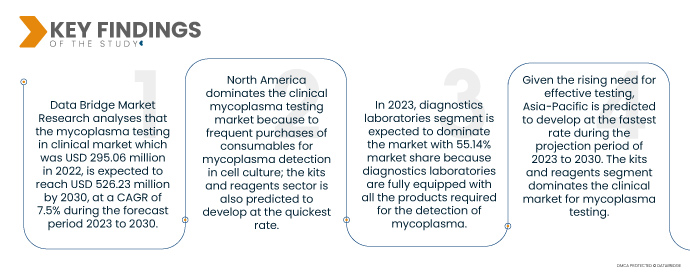
Data Bridge Market Research analiza que el Pruebas de micoplasma en el mercado clínico que fue de 295,06 millones de dólares en 2022, se espera que alcance los 526,23 millones de dólares en 2030, con una tasa compuesta anual del 7,5% durante el período previsto de 2023 a 2030. Se desarrollaron PCR y qPCR de alta sensibilidad, lo que aumentó la especificidad de las pruebas para más de 90 micoplasmas. especies. La prueba incluye una variedad de productos como lectores, ensayos, instrumentos, kits y reactivos, todos los cuales contribuyen al crecimiento del mercado. Un gran número de participantes del mercado participan en la producción de dispositivos y consumibles utilizados para la identificación de diversas especies de micoplasmas, lo que allana el camino para la expansión del mercado.
El creciente número de enfermedades crónicas es Se espera que impulse la tasa de crecimiento del mercado.
Actualmente, diversas enfermedades infecciosas están aumentando debido a la mala higiene y a otros factores. Se sabe que Mycoplasma causa una variedad de enfermedades al atacar varias partes del cuerpo. El micoplasma se ha relacionado con la uretritis, la enfermedad de Crohn, la neumonía y las enfermedades neurológicas, entre otras cosas. Debido a que el micoplasma es la causa principal de muchas enfermedades, crece la necesidad de realizar pruebas clínicas para proporcionar el tratamiento adecuado en el momento adecuado. Como resultado, el creciente número de enfermedades está impulsando la expansión de las pruebas de micoplasmas en el mercado clínico a nivel mundial.
Alcance del informe y segmentación del mercado
|
Métrica de informe
|
Detalles
|
|
Período de pronóstico
|
2023 a 2030
|
|
Año base
|
2022
|
|
Años históricos
|
2021 (Personalizable para 2015-2020)
|
|
Unidades Cuantitativas
|
Ingresos en millones de dólares, volúmenes en unidades, precios en dólares
|
|
Segmentos cubiertos
|
Productos (kits y reactivos, instrumentos, servicios), técnica (técnicas de cultivo microbiano/ensayo directo, reacción en cadena de la polimerasa, ELISA, tinción de ADN/ensayo indirecto, métodos enzimáticos), área de enfermedad (respiratoria, urogenital, gastrointestinal, musculoesquelética, cardiovascular, otras ), Usuario final (Laboratorios de diagnóstico, Hospitales)
|
|
Países cubiertos
|
Estados Unidos, Canadá y México en Norteamérica, Alemania, Francia, Reino Unido, Países Bajos, Suiza, Bélgica, Rusia, Italia, España, Turquía, Resto de Europa en Europa, China, Japón, India, Corea del Sur, Singapur, Malasia, Australia, Tailandia, Indonesia, Filipinas, Resto de Asia-Pacífico (APAC) en Asia-Pacífico (APAC), Arabia Saudita, Emiratos Árabes Unidos, Sudáfrica, Egipto, Israel, Resto de Medio Oriente y África (MEA) como parte de Medio Oriente y África (MEA), Brasil, Argentina y Resto de Sudamérica como parte de Sudamérica
|
|
Actores del mercado cubiertos
|
AB ANALITICA srl (Italia), bioMérieux SA (Francia), ELITechGroup (Francia), Liofilchem Srl (Italia), Agilent Technologies, Inc. (EE.UU.), PromoCell GmbH (Alemania), F. Hoffmann-La Roche Ltd (Suiza), OSANG Healthcare (Corea del Sur), Sacace Biotechnologies Srl (Italia), Lonza (Suiza), Merck KGaA (Alemania), Seegene Inc. (Corea del Sur), Clongen Laboratories, LLC (EE.UU.), Bio-Rad Laboratories, Inc. (EE.UU. ), Charles River Laboratories (EE.UU.), Bionique Testing Laboratories, Inc. (EE.UU.) y ZEAKON Diagnostics (EE.UU.)
|
|
Puntos de datos cubiertos en el informe
|
Además de los conocimientos del mercado, como el valor de mercado, la tasa de crecimiento, los segmentos de mercado, la cobertura geográfica, los actores del mercado y el escenario del mercado, el informe de mercado elaborado por el equipo de investigación de mercado de Data Bridge incluye análisis de expertos en profundidad, epidemiología de pacientes y análisis de tuberías. , análisis de precios y marco regulatorio
|
Análisis de segmentos:
Las pruebas globales de micoplasma en el mercado clínico se clasifican en cuatro segmentos notables que se basan en productos, técnicas, áreas de enfermedades y usuarios finales.
- Según los productos, las pruebas de micoplasma en el mercado clínico se segmentan en kits y reactivos, instrumentos y servicios. En 2023, se espera que el segmento de kits y reactivos domine el mercado con una participación de mercado del 54,31% porque los kits y reactivos se compran repetidamente como productos para la detección de micoplasmas.
- Según la técnica, las pruebas de micoplasma en el mercado clínico se segmentan en técnicas de cultivo microbiano/ensayo directo, reacción en cadena de la polimerasa, ELISA, tinción de ADN/ensayo indirecto y métodos enzimáticos. En 2023, se espera que el segmento de técnicas de cultivo microbiano/ensayo directo domine el mercado con una participación de mercado del 36,42% porque estas técnicas son rentables y altamente específicas cuando se obtiene un resultado positivo.
- Según el área de la enfermedad, las pruebas de micoplasma en el mercado clínico se segmentan en respiratorias, urogenitales, gastrointestinales, cardiovasculares, musculoesqueléticas y otras. En 2023, se espera que el segmento respiratorio domine el mercado con un 36,37% porque el micoplasma afecta a niños y adultos con neumonías de leves a graves.
El segmento respiratorio dominará el segmento de áreas de enfermedades de las pruebas de micoplasma en el mercado clínico.
El segmento respiratorio surgirá como el segmento dominante en el área de enfermedades con aproximadamente una participación de mercado del 37,00%. Esto se debe al creciente número de actividades de desarrollo de infraestructura en el mercado, especialmente en las economías en desarrollo. Además, el crecimiento y la expansión de la industria de la salud en todo el mundo impulsarán aún más el crecimiento de este segmento.
- Según el usuario final, las pruebas de micoplasma en el mercado clínico se segmentan en hospitales y laboratorios de diagnóstico. En 2023, se espera que el segmento de laboratorios de diagnóstico domine el mercado con una participación de mercado del 55,14% porque los laboratorios de diagnóstico están completamente equipados con todos los productos necesarios para la detección de micoplasmas y la presencia de profesionales capacitados.
El segmento de laboratorios de diagnóstico dominará el segmento de usuarios finales de las pruebas de micoplasma en el mercado clínico.
El segmento de centros de laboratorios de diagnóstico surgirá como el segmento dominante de usuarios finales. Esto se debe al creciente número de laboratorios de diagnóstico en el mercado, especialmente en las economías en desarrollo. Además, el crecimiento y la expansión de los servicios de desarrollo de investigación a escala global impulsarán aún más el crecimiento de este segmento.
Principales actores
Data Bridge Market Research reconoce a las siguientes empresas como los principales actores del mercado: AB ANALITICA srl (Italia), bioMérieux SA (Francia), ELITechGroup (Francia), Liofilchem Srl (Italia), Agilent Technologies, Inc. (EE. UU.), PromoCell GmbH ( Alemania), F. Hoffmann-La Roche Ltd (Suiza), OSANG Healthcare (Corea del Sur), Sacace Biotechnologies Srl (Italia), Lonza (Suiza), Merck KGaA (Alemania), Seegene Inc. (Corea del Sur), Clongen Laboratories, LLC (EE. UU.), Bio-Rad Laboratories, Inc. (EE. UU.), Charles River Laboratories (EE. UU.), Bionique Testing Laboratories, Inc. (EE. UU.) y ZEAKON Diagnostics (EE. UU.).
El desarrollo del mercado
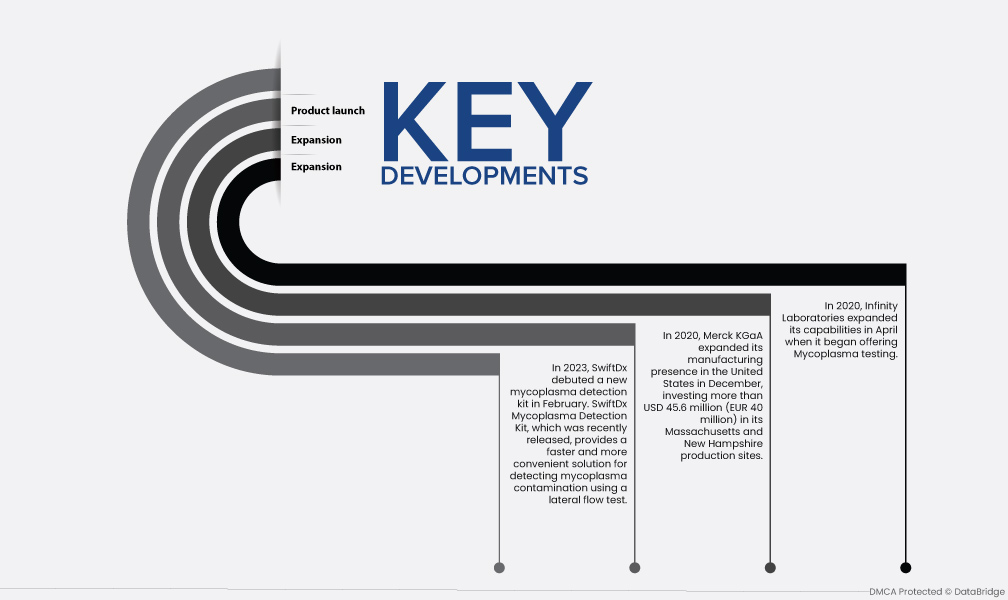
- En 2023, SwiftDx presentó un nuevo kit de detección de micoplasmas en febrero. El kit de detección de micoplasmas SwiftDx, que se lanzó recientemente, proporciona una solución más rápida y conveniente para detectar la contaminación por micoplasmas mediante una prueba de flujo lateral.
- En 2020, Merck KGaA amplió su presencia manufacturera en Estados Unidos en diciembre, invirtiendo más de 45,6 millones de dólares (40 millones de euros) en sus plantas de producción de Massachusetts y New Hampshire. Los sitios fueron diseñados para generar una variedad de productos de fabricación biofarmacéuticos y al mismo tiempo aumentar la capacidad de producción de la empresa.
- En 2020, Infinity Laboratories amplió sus capacidades en abril cuando comenzó a ofrecer pruebas de Mycoplasma. Las capacidades de los principales actores del mercado mejorarán como consecuencia de la implementación de estrategias clave como expansión, asociaciones, adquisiciones y otras, lo que dará como resultado la expansión del mercado.
Análisis Regional
Geográficamente, los países cubiertos en el informe de mercado son EE.UU., Canadá y México en América del Norte, Alemania, Francia, Reino Unido, Países Bajos, Suiza, Bélgica, Rusia, Italia, España, Turquía, Resto de Europa en Europa, China, Japón, India. , Corea del Sur, Singapur, Malasia, Australia, Tailandia, Indonesia, Filipinas, Resto de Asia-Pacífico (APAC), Arabia Saudita, Emiratos Árabes Unidos, Sudáfrica, Egipto, Israel, Resto de Medio Oriente y África (MEA) como parte de Medio Oriente y África (MEA), Brasil, Argentina y Resto de Sudamérica como parte de Sudamérica.
Según el análisis de investigación de mercado de Data Bridge:
América del Norte es la región dominante en las pruebas de micoplasma en el ámbito clínico. mercado durante el período previsto 2023 a 2030
América del Norte domina el mercado de pruebas clínicas de micoplasmas debido a las frecuentes compras de consumibles para la detección de micoplasmas en cultivos celulares; También se prevé que el sector de kits y reactivos se desarrolle al ritmo más rápido.
Se estima que Asia-Pacífico es la región de más rápido crecimiento en el pruebas de micoplasma en clínica mercado en el período previsto 2023 a 2030
Dada la creciente necesidad de pruebas eficaces, se prevé que Asia-Pacífico se desarrolle al ritmo más rápido durante el período de proyección de 2023 a 2030. El segmento de kits y reactivos domina el mercado clínico para pruebas de micoplasma.
Para obtener información más detallada sobre las pruebas de micoplasma en el mercado clínico. informe, haga clic aquí – https://www.databridgemarketresearch.com/reports/global-mycoplasma-testing-in-clinical-market