North America Dermatology Treatment Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1.80 Billion
USD
3.60 Billion
2024
2032
USD
1.80 Billion
USD
3.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.80 Billion | |
| USD 3.60 Billion | |
|
|
|
|
North America Dermatology Treatment Devices Market Segmentation, by type (Push Button Safety Lancet, Pressure Activated Safety Lancet, Side Button Safety Lancet), by application (Blood Glucose Testing, Hemoglobin Testing, Cholesterol Testing, Coagulation Testing), by end user (Hospitals & Clinics, Diagnostic Centers and Pathology Laboratories, Home Diagnostics, Others)- Industry Trends and Forecast to 2032
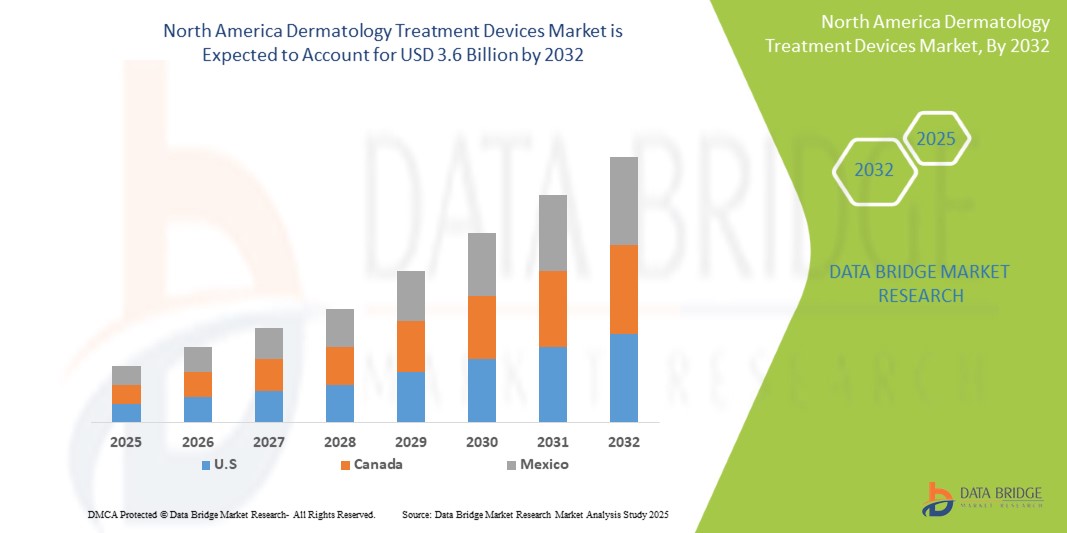
North America Dermatology Treatment Devices Market Size
- The North America Dermatology Treatment Devices Market was valued atUSD1.8 Billionin 2024and is expected to reachUSD3.6 Billionby 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at aCAGR of 8.9%,primarily driven by the increasing prevalence of chronic diseases
- Key drivers of the North America Dermatology Treatment Devices Market include the rising prevalence of chronic diseases like diabetes, growing awareness of needle-stick injuries, and the demand for safer and more efficient blood sampling devices.
North America Dermatology Treatment Devices Market Analysis
- The increasing focus on minimizing needle-stick injuries in healthcare settings is driving the adoption of safety lancets as a safer alternative for blood sampling.
- The rise in chronic diseases, especially diabetes, is fueling the demand for blood glucose monitoring devices, including safety lancets, for at-home and clinical use.
- For instance, Innovations in lancet design, such as push-button mechanisms and pressure-activated features, are enhancing user comfort and safety, further contributing to market growth..
- Increasing awareness regarding safe and hygienic blood sampling techniques in both medical and home care settings is promoting the use of safety lancets.
- As healthcare infrastructure improves, especially in developing regions, the demand for advanced medical devices, including safety lancets, is expected to rise significantly.
Report Scope and North America Dermatology Treatment Devices Market Segmentation
|
Attributes |
North America Dermatology Treatment Devices Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Dermatology Treatment Devices Market Trends
“integration of smart technology and connectivity features”
- Enhanced Patient Monitoring and Data Collection: Smart safety lancets are equipped with features like integrated biosensors and Bluetooth connectivity, enabling real-time data transmission to digital health platforms. This facilitates continuous monitoring of blood parameters and supports proactive healthcare interventions.
- Improved Patient Compliance and Engagement: By connecting to mobile applications, these devices provide users with reminders, usage statistics, and health insights, thereby encouraging regular testing and adherence to treatment plans.
- Support for Remote Healthcare Services: The integration of smart technology aligns with the growing demand for telemedicine and home healthcare services, allowing healthcare providers to remotely monitor patients' health status and make informed decisions without the need for in-person visits.
North America Dermatology Treatment Devices Market Dynamics
Driver
“rising prevalence of chronic diseases”
- Increased Demand for Regular Blood Sampling: Chronic diseases require frequent blood tests to monitor parameters like glucose levels, cholesterol, and hemoglobin, leading to a higher need for safe and efficient blood sampling devices.
- Enhanced Patient Safety and Compliance: Safety lancets are designed to minimize the risk of needlestick injuries and cross-contamination, encouraging patients to adhere to regular testing schedules.
- Alignment with Healthcare Regulations: The adoption of safety lancets supports healthcare facilities in complying with stringent safety standards and infection control protocols, promoting their widespread use in clinical settings.
Opportunity
“development of sustainable and eco-friendly safety lancets”
- Environmental Impact Reduction: With increasing global emphasis on sustainability, manufacturers are innovating safety lancets using biodegradable materials and recyclable packaging to minimize medical waste.
- Regulatory Compliance and Market Demand: Adhering to environmental regulations and responding to consumer demand for eco-conscious products can enhance brand reputation and market share.
- Alignment with Healthcare Sustainability Goals: Integrating sustainable practices in product development aligns with the broader healthcare industry's shift towards greener solutions, potentially opening new market segments and partnerships.
Restraint/Challenge
“high cost of advanced lancet technologies”
- Increased Production Costs: Advanced safety lancets often incorporate features like adjustable penetration depths and ergonomic designs, which enhance user comfort and safety but also increase manufacturing expenses.
- Limited Accessibility in Developing Regions: The elevated costs of these advanced devices can make them less accessible in developing countries, where healthcare budgets are constrained, and cost-effective alternatives are preferred.
North America Dermatology Treatment Devices Market Scope
The market is segmented on the basis application, type, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Application |
|
|
By Type |
|
|
By End User |
|
North America Dermatology Treatment Devices Market Regional Analysis
“North America is the Dominant Region in the North America Dermatology Treatment Devices Market”
- High Prevalence of Chronic Diseases: The region exhibits a significant incidence of chronic conditions like diabetes, cardiovascular diseases, and cancer. For instance, in the United States, approximately 6 out of 10 adults have at least one chronic disease, necessitating regular blood sampling for management and monitoring.
- Robust Healthcare Infrastructure and Reimbursement Policies: North America boasts advanced healthcare facilities and favorable reimbursement policies, facilitating the adoption of safety lancets. In Canada, initiatives such as the Disability Tax Credit for insulin users underscore the region's commitment to supporting individuals with chronic conditions
- Technological Advancements and Market Growth: The region is witnessing continuous innovation in safety lancet technologies, including features like adjustable penetration depths and ergonomic designs. This progression, coupled with a substantial patient base and increasing healthcare expenditure, positions North America to account for a significant share of the market's growth
“Asia-Pacific is Projected to Register the Highest Growth Rate in the North America Dermatology Treatment Devices Market”
- High Prevalence of Diabetes: Over 60% of the Asian population is living with diabetes, with China and India accounting for nearly half of this number. The Western Pacific region alone has more than 138.2 million people with diabetes, a number expected to rise to 201.8 million by 2035. This surge in diabetic cases necessitates frequent blood sampling, driving demand for safety lancets.
- Government Initiatives Promoting Screening: Countries like India and China are implementing large-scale health initiatives to combat chronic diseases. For instance, India's Union Health Ministry launched a program in 2023 aiming to screen 75 million people for hypertension and diabetes by 2025. Similarly, China's 'Healthy China 2030' initiative focuses on integrated diabetes management across urban and rural areas, further increasing the need for diagnostic tools like safety lancets.
- Advancements in Healthcare Infrastructure: Rapid urbanization and improvements in healthcare services across Asia-Pacific are enhancing access to medical diagnostics. The rising incidence of infectious diseases, such as malaria, dengue, and chikungunya, which require diagnostic testing, is also contributing to the increased demand for safety lancets in the region.
North America Dermatology Treatment Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd
- Becton, Dickinson and Company (BD)
- Terumo Medical Corporation
- Bayer AG
- HTL-STREFA S.A.
- Sarstedt AG & Co. KG
- Improve Medical Technology Co. Ltd
- Ypsomed AG
- Greiner Bio-One International GmbH
- Owen Mumford Ltd
- Smiths Medical
- Nipro Corporation
- Cardinal Health, Inc.
- Arkray Inc.
- Medline Industries, Inc.
Latest Developments in North America Dermatology Treatment Devices Market
- In 2021, FUJIFILM Corporation opened NURA, a medical screening center focusing on cancer screening in Bangalore, India. This medical screening center is operated by FUJIFILM DKH LLP (FUJIFILM DKH) and Dr. Kutty’s Healthcare (DKH). FUJIFILM DKH LLP (FUJIFILM DKH) is a joint venture of FUJIFILM and Dr. Kutty’s Healthcare (DKH), which runs hospitals and screening centers in India and the Middle East.
- In 2023, Astellas Pharma announced that it has entered into an agreement with Roche Diabetes Care Japan Co., Ltd. for the development and commercialization of Roche Diabetes Care’s world-renowned Accu-Chek Guide Me blood glucose monitoring system with advanced accuracy as a combined medical product with BlueStar.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.