North America Medical Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
4,694.22 Million
USD
6,882.84 Million
2022
2030
USD
4,694.22 Million
USD
6,882.84 Million
2022
2030
| 2023 –2030 | |
| USD 4,694.22 Million | |
| USD 6,882.84 Million | |
|
|
|
|
Nordamerikanischer Markt für medizinische Geräte, nach Produkt ( Beatmungsgerät , Spirometer, Sauerstoffkonzentratoren, Anästhesiegeräte und CPAP/BIPAP), Modus (tragbar, Tischgerät und Standalone), Anwendung (Diagnose und Therapie), Einrichtung (groß, klein und mittel), Endbenutzer (Krankenhaus, ambulante chirurgische Zentren, Fachkliniken, Langzeitpflegezentren, Rehabilitationszentren, Einrichtungen für die häusliche Pflege), Vertriebskanal (Direktvertrieb und Drittanbieter) – Branchentrends und Prognose bis 2030.
Marktanalyse und Größe für medizinische Geräte in Nordamerika
Nach Angaben der Centers for Disease Control and Prevention (CDC) leiden in den USA etwa 25 Millionen Menschen (Erwachsene und Kinder) an Asthma. Die geschätzte Inzidenzrate liegt bei Erwachsenen bei 8,4 % und bei Kindern bei 8,1 %. Schätzungsweise 16 Millionen Erwachsene in den USA sind mit COPD diagnostiziert worden, weitere 16 Millionen Menschen leiden an nicht diagnostizierter COPD. Die zunehmende Inzidenz von Atemwegserkrankungen ist ein Wachstumstreiber für den Markt für medizinische Geräte in Nordamerika.
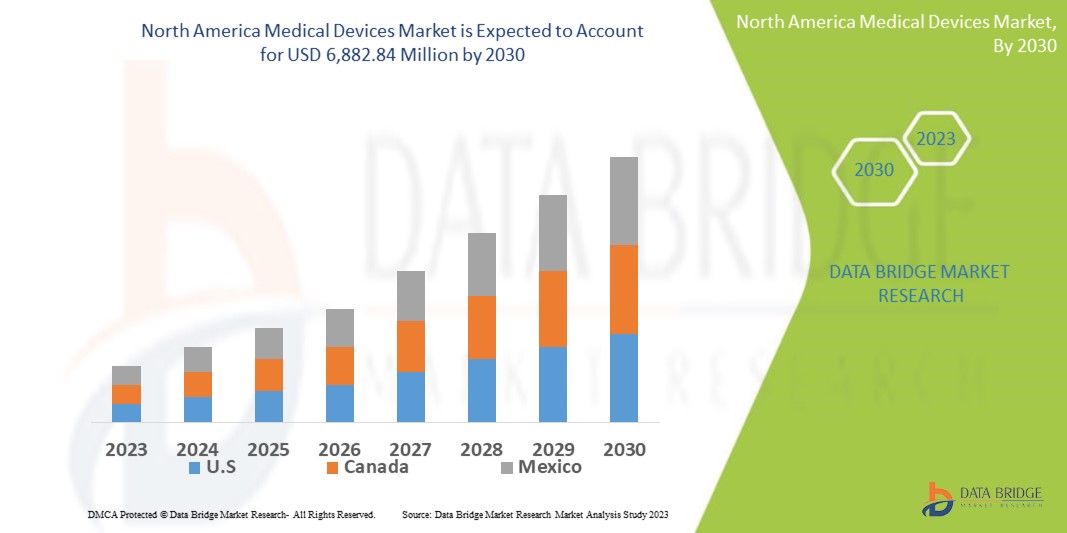
Data Bridge Market Research analysiert, dass der nordamerikanische Markt für medizinische Geräte, der im Jahr 2022 4.694,22 Millionen USD betrug, bis 2030 voraussichtlich 6.882,84 Millionen USD erreichen wird und im Prognosezeitraum 2023–2030 eine durchschnittliche jährliche Wachstumsrate von 4,9 % aufweisen wird. Dies zeigt den Marktwert. „Beatmungsgeräte“ dominieren das Produktsegment des Marktes für medizinische Geräte aufgrund der wachsenden Nachfrage nach besseren Behandlungsmethoden. Neben Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und Hauptakteure enthalten die von Data Bridge Market Research zusammengestellten Marktberichte auch eingehende Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
Marktumfang und -segmentierung für medizinische Geräte in Nordamerika
|
Berichtsmetrik |
Details |
|
Prognosezeitraum |
2023 bis 2030 |
|
Basisjahr |
2022 |
|
Historische Jahre |
2021 (anpassbar auf 2015–2020) |
|
Quantitative Einheiten |
Umsatz in Millionen USD, Mengen in Einheiten und Preise in USD |
|
Abgedeckte Segmente |
Produkt (Beatmungsgerät, Spirometer , Sauerstoffkonzentratoren, Anästhesiegeräte und CPAP/BIPAP), Modus (tragbar, Tischgerät und Standalone), Anwendung (Diagnostik und Therapie), Einrichtung (groß, klein und mittel), Endnutzer (Krankenhaus, ambulante chirurgische Zentren, Fachkliniken, Langzeitpflegezentren, Rehabilitationszentren, Einrichtungen für häusliche Pflege), Vertriebskanal (Direktvertrieb und Drittanbieter) |
|
Abgedeckte Länder |
USA, Kanada und Mexiko |
|
Abgedeckte Marktteilnehmer |
GE Healthcare (USA), Koninklijke Philips NV (Niederlande), Medtronic (Irland), Drägerwerk AG & Co. KGaA (Deutschland), VYAIRE (USA), Getinge AB (Schweden), Smiths Medical Inc. (ein Teil der Smiths Group plc.) (USA), NDD Medical Technologies (Schweiz), ResMed (USA), Invacare Corporation (USA), NIDEK MEDICAL (Japan), O2 CONCEPTS, LLC (USA), Teijin Limited (Japan), GCE Healthcare (Großbritannien), Inogen, Inc (USA), Teleflex Incorporated (USA), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China), MGC Diagnostics Corporation (USA), HILL-ROM (USA), Drive DeVilbiss Healthcare Inc. (USA), Midmark Corporation (USA), CAIRE Inc. (USA), GCE Group (Schweden), Fisher & Paykel Healthcare Limited (Neuseeland) und Schiller (Schweiz) |
|
Marktchancen |
|
Marktdefinition
Medizinprodukte sind alle Geräte, Maschinen, Hilfsmittel, Apparate, Instrumente, Implantate, Reagenzien für die In-vitro-Verwendung, Materialien, Software oder andere verwandte oder ähnliche Artikel, die vom Hersteller für die Verwendung einzeln oder in Kombination für medizinische Zwecke vorgesehen sind. Medizinprodukte werden zur Linderung, Behandlung, Überwachung, Vorbeugung oder Diagnose von Krankheiten eingesetzt. Darüber hinaus werden Medizinprodukte auch zur Unterstützung, Änderung, zum Ersatz oder zur Untersuchung physiologischer oder anatomischer Prozesse eingesetzt. Darüber hinaus werden Medizinprodukte auch zur Lebenserhaltung oder -unterstützung eingesetzt. Medizinprodukte umfassen eine breite Palette von Produkten mit unterschiedlicher Anwendung und Komplexität. Beispiele sind unter anderem Röntgengeräte, Beatmungsgeräte, diagnostische Medizinprodukte, therapeutische Medizinprodukte und Sauerstoffkonzentratoren.
Marktdynamik für Medizinprodukte in Nordamerika
Treiber
- Zunehmende Verbreitung chronischer Krankheiten
Die steigende Verbreitung chronischer Krankheiten wie Herz-Kreislauf-Erkrankungen, Diabetes und Atemwegserkrankungen treibt die Nachfrage nach medizinischen Geräten an. Diese Geräte helfen bei der Diagnose, Überwachung und Behandlung dieser Erkrankungen und verbessern die Behandlungsergebnisse und die Lebensqualität der Patienten.
- Wachsendes Bewusstsein und Patientenermächtigung
Patienten werden sich der verfügbaren Behandlungsmöglichkeiten immer bewusster und beteiligen sich aktiv an Entscheidungen im Gesundheitswesen. Dieses gesteigerte Bewusstsein und die damit verbundene Selbstbestimmung treiben die Nachfrage nach medizinischen Geräten voran, die die Behandlungsergebnisse der Patienten verbessern und eine personalisierte Betreuung bieten können.
Gelegenheiten
- Steigende Nachfrage nach häuslicher Gesundheitsfürsorge
Der Trend hin zu häuslicher Gesundheitsfürsorge stellt für Hersteller medizinischer Geräte eine Chance dar. Dank technologischer Fortschritte können Patienten mit tragbaren Monitoren, Telemedizinlösungen und Heimdiagnosetools bequem von zu Hause aus versorgt werden. Dieser Trend eröffnet Medizingeräteherstellern neue Märkte und Einnahmequellen.
- Aufstieg der digitalen Gesundheit und vernetzter Geräte
Die Integration digitaler Gesundheitstechnologien wie Internet der Dinge (IoT), künstliche Intelligenz (KI) und Big-Data-Analyse revolutioniert die Gesundheitsbranche. Vernetzte Geräte und Lösungen zur Patientenfernüberwachung bieten Möglichkeiten für eine verbesserte Patientenversorgung, Echtzeit-Datenanalyse und personalisierte Behandlungspläne. Medizintechnikunternehmen können von diesem Trend profitieren, indem sie innovative vernetzte Geräte und Softwarelösungen entwickeln.
Einschränkungen/Herausforderungen
- Technologische Herausforderungen und Produktkomplexität
Die Entwicklung und Vermarktung komplexer medizinischer Geräte, wie beispielsweise hochentwickelter Bildgebungssysteme oder implantierbarer Geräte, kann technische Herausforderungen mit sich bringen. Die hohen Kosten, das technische Know-how und die langen Entwicklungszeiten, die mit solchen Geräten verbunden sind, können für Unternehmen eine Hürde darstellen.
- Strenges regulatorisches Umfeld
Während gesetzliche Standards die Patientensicherheit und Produktqualität gewährleisten, können die strengen gesetzlichen Anforderungen für Hersteller medizinischer Geräte eine Herausforderung darstellen. Die Einholung von Genehmigungen und Freigaben von Aufsichtsbehörden wie der US-amerikanischen Food and Drug Administration (FDA) kann zeitaufwändig und teuer sein, was die Produkteinführung verzögert und die Kosten erhöht.
Dieser Marktbericht für Medizinprodukte in Nordamerika enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-/Exportanalysen, Produktionsanalysen, Optimierung der Wertschöpfungskette, Marktanteilen, dem Einfluss inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neue Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografischen Expansionen und technologischen Innovationen auf dem Markt. Um weitere Informationen zum Markt für Medizinprodukte in Nordamerika zu erhalten, wenden Sie sich an Data Bridge Market Research, um einen Analystenbericht zu erhalten. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Jüngste Entwicklungen
- Im Mai 2021 kündigte Medtronic die Einführung seines roboterassistierten Chirurgiesystems Hugo in den USA an.
- Im März 2021 erhielt die Johnson & Johnson-Tochter Janssen Pharmaceuticals die FDA-Zulassung für ihren COVID-19-Impfstoff, der in Zusammenarbeit mit Janssen Vaccines & Prevention BV entwickelt wurde.
Marktumfang für Medizinprodukte in Nordamerika
Der nordamerikanische Markt für medizinische Geräte ist nach Produkt, Modus, Anwendung, Einrichtung, Endbenutzer und Vertriebskanal segmentiert. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, die ihnen bei der strategischen Entscheidungsfindung zur Identifizierung der wichtigsten Marktanwendungen helfen.
Produkt
- Ventilator
- Spirometer
- Sauerstoffkonzentratoren
- Anästhesiegeräte
- CPAP/BIPAP
Modus
- Tragbar
- Tischplatte
- Standalone
Anwendung
- Diagnostik
- Therapeutisch
Einrichtung
- Groß
- Klein
- Medium
Endbenutzer
- Krankenhaus
- Ambulante Chirurgische Zentren
- Spezialkliniken
- Langzeitpflegezentren
- Rehabilitationszentren
- Einstellungen für die häusliche Pflege
Vertriebskanal
- Direktvertrieb
- Drittanbieter-Distributor
Regionale Analyse/Einblicke zum nordamerikanischen Markt für medizinische Geräte
Der nordamerikanische Markt für medizinische Geräte wird analysiert und es werden Einblicke in die Marktgröße und Trends nach Land, Produkt, Modus, Anwendung, Einrichtung, Endbenutzern und Vertriebskanal wie oben angegeben bereitgestellt.
Die im nordamerikanischen Marktbericht für medizinische Geräte abgedeckten Länder sind die USA, Kanada und Mexiko.
Die USA dominieren den nordamerikanischen Markt für medizinische Geräte aufgrund der starken Basis an Gesundheitseinrichtungen, der starken Präsenz wichtiger Marktteilnehmer, der außergewöhnlichen Gesundheitsinfrastruktur und der großen Zahl chronisch Kranker.
Der Länderabschnitt des Berichts enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Regulierung auf dem Inlandsmarkt, die sich auf die aktuellen und zukünftigen Trends des Marktes auswirken. Datenpunkte wie Downstream- und Upstream-Wertschöpfungskettenanalysen, technische Trends und Porters Fünf-Kräfte-Analyse sowie Fallstudien sind einige der Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Bei der Bereitstellung von Prognoseanalysen der Länderdaten werden auch die Präsenz und Verfügbarkeit globaler Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken, die Auswirkungen inländischer Zölle und Handelsrouten berücksichtigt.
Wachstum der installierten Basis der Gesundheitsinfrastruktur und Durchdringung mit neuen Technologien
Der nordamerikanische Markt für Medizinprodukte bietet Ihnen außerdem eine detaillierte Marktanalyse zum Wachstum der Gesundheitsausgaben für Investitionsgüter in jedem Land, zur installierten Basis verschiedener Produktarten für den nordamerikanischen Markt für Medizinprodukte, zum Einfluss der Technologie anhand von Lebenslinienkurven und zu Änderungen der regulatorischen Szenarien im Gesundheitswesen und deren Auswirkungen auf den Markt für Medizinprodukte. Diese Daten sind für den historischen Zeitraum 2015–2020 verfügbar.
Wettbewerbsumfeld und Marktanteilsanalyse für medizinische Geräte in Nordamerika
Die Wettbewerbslandschaft des nordamerikanischen Marktes für medizinische Geräte liefert Einzelheiten zu den Wettbewerbern. Zu den enthaltenen Einzelheiten gehören Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang, Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen in Bezug auf den nordamerikanischen Markt für medizinische Geräte.
Zu den wichtigsten Akteuren auf dem nordamerikanischen Markt für medizinische Geräte zählen:
- GE Healthcare (USA)
- Koninklijke Philips NV (Niederlande)
- Medtronic (Irland)
- Drägerwerk AG & Co. KGaA (Deutschland)
- VYAIRE (USA)
- Getinge AB (Schweden)
- Smiths Medical Inc. (Teil der Smiths Group plc.) (USA)
- NDD Medical Technologies (Schweiz)
- ResMed (USA)
- Invacare Corporation (USA)
- NIDEK MEDICAL (Japan)
- O2 CONCEPTS, LLC (USA)
- Teijin Limited (Japan)
- GCE Healthcare (Großbritannien)
- Inogen, Inc (U.S.)
- Teleflex Incorporated (U.S.)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- MGC Diagnostics Corporation (U.S.)
- HILL-ROM (U.S.)
- Drive DeVilbiss Healthcare Inc. (U.S.)
- Midmark Corporation (U.S.)
- CAIRE Inc. (U.S.)
- GCE Group (Sweden)
- Fisher & Paykel Healthcare Limited (New Zealand)
- Schiller (Switzerland)
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.