North America Cardiac Monitoring Cardiac Rhythm Management Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
2.42 Billion
USD
3.29 Billion
2024
2032
USD
2.42 Billion
USD
3.29 Billion
2024
2032
| 2025 –2032 | |
| USD 2.42 Billion | |
| USD 3.29 Billion | |
|
|
|
|
Marktsegmentierung für Geräte zur Herzüberwachung und Herzrhythmuskontrolle in Nordamerika nach Produkt (EKG-Geräte, implantierbare Loop-Recorder, Geräte zur Überwachung des Herzzeitvolumens (COM), Ereignismonitore, mobile kardiale Telemetrieüberwachung (MCT/MCOT), intelligente tragbare EKG-Monitore, Defibrillatoren, Herzschrittmacher und Geräte zur kardialen Resynchronisationstherapie (CRT), Endbenutzer (Krankenhäuser, häusliche und ambulante Pflegeeinrichtungen und andere) – Branchentrends und Prognose bis 2032
Marktgröße für Geräte zur Herzüberwachung und Herzrhythmuskontrolle in Nordamerika
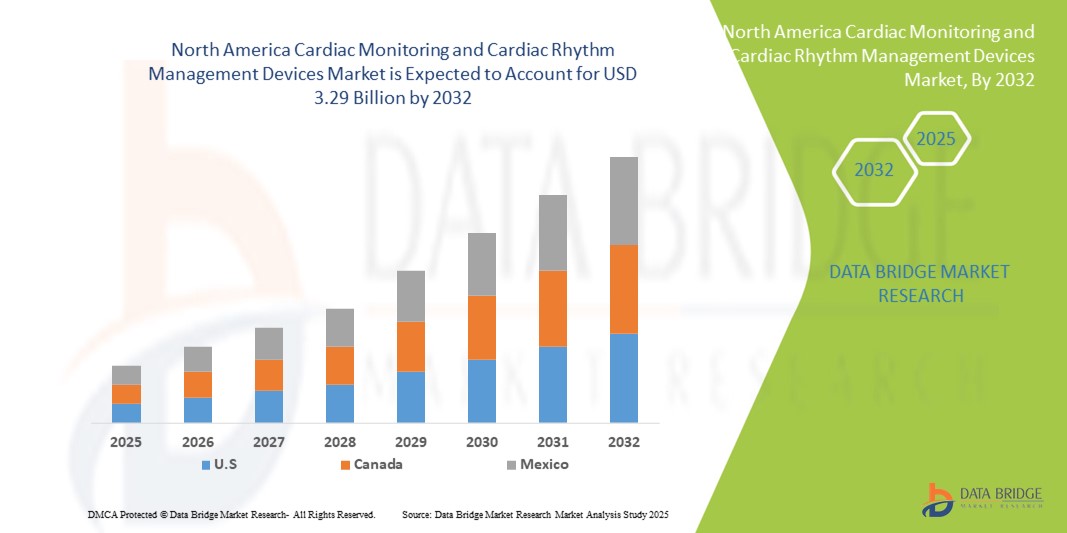
- Der nordamerikanische Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle hatte im Jahr 2024 einen Wert von 2,42 Milliarden US-Dollar und dürfte bis 2032 3,29 Milliarden US-Dollar erreichen , bei einer CAGR von 3,90 % im Prognosezeitraum.
- Das Marktwachstum wird maßgeblich durch die zunehmende Verbreitung von Herz-Kreislauf-Erkrankungen sowie den steigenden Bedarf an kontinuierlicher Patientenüberwachung und rechtzeitigen therapeutischen Interventionen vorangetrieben. Fortschritte bei digitalen Gesundheitstechnologien, darunter implantierbare Geräte, tragbare Monitore und Fernüberwachungssysteme, fördern die Akzeptanz in Krankenhäusern und der häuslichen Gesundheitsversorgung.
- Darüber hinaus steigert das wachsende Bewusstsein von Gesundheitsdienstleistern und Patienten für die Früherkennung von Arrhythmien, Herzinsuffizienz und anderen Herzerkrankungen die Nachfrage nach Lösungen zur Herzüberwachung und zum Herzrhythmusmanagement. Kontinuierliche Innovationen bei der Miniaturisierung von Geräten, der Konnektivität und der Integration in Telemedizin-Plattformen verbessern die Patientenergebnisse. Diese konvergierenden Faktoren beschleunigen die Einführung von Geräten zur Herzüberwachung und zum Herzrhythmusmanagement und fördern damit das Wachstum der Branche erheblich.
Marktanalyse für Geräte zur Herzüberwachung und Herzrhythmuskontrolle in Nordamerika
- Geräte zur Herzüberwachung und zum Herzrhythmusmanagement, einschließlich implantierbarer und tragbarer Lösungen, sind zunehmend wichtige Bestandteile moderner Gesundheitssysteme im klinischen und häuslichen Umfeld, da sie eine kontinuierliche Überwachung in Echtzeit, die Früherkennung von Herzanomalien und eine nahtlose Integration mit digitalen Gesundheitsplattformen ermöglichen.
- Die steigende Nachfrage nach Geräten zur Herzüberwachung und Herzrhythmuskontrolle wird vor allem durch die zunehmende Verbreitung von Herz-Kreislauf-Erkrankungen, das steigende Bewusstsein für die Herzgesundheit und die zunehmende Nutzung fortschrittlicher medizinischer Technologien zur kontinuierlichen Patientenüberwachung angetrieben.
- Die USA dominierten den Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle mit dem größten Umsatzanteil von 74,8 % im Jahr 2024. Dies ist auf die fortschrittliche Gesundheitsinfrastruktur, die hohe Prävalenz von Herzerkrankungen und die starke Verbreitung innovativer Geräte wie implantierbare Kardioverter-Defibrillatoren (ICDs), Herzschrittmacher und tragbare Herzmonitore zurückzuführen. Der US-Markt profitiert von häufigen Produkteinführungen, technologischen Fortschritten und soliden Erstattungssystemen, die einen breiten Patientenzugang zu modernen Lösungen für die Herzversorgung gewährleisten.
- Kanada wird im Prognosezeitraum voraussichtlich das am schnellsten wachsende Land im Markt für Herzüberwachungs- und Herzrhythmusmanagementgeräte sein und voraussichtlich eine starke jährliche Wachstumsrate (CAGR) von 7,8 % verzeichnen. Dies wird durch zunehmende staatliche Initiativen zur Verbesserung der Herzversorgung, einen verbesserten Zugang zu Krankenhäusern und Fachkliniken sowie ein wachsendes Bewusstsein für präventive Kardiologie und Fernüberwachung von Patienten vorangetrieben. Die Einführung tragbarer Geräte der nächsten Generation und Telemonitoring-Technologien beschleunigt das Marktwachstum im Land zusätzlich.
- Das Segment Herzschrittmacher dominierte den Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle mit dem größten Marktanteil von 32,5 % im Jahr 2024, was auf die weit verbreitete klinische Anwendung zur Behandlung von Bradykardie und anderen Herzrhythmusstörungen zurückzuführen ist.
Berichtsumfang und Marktsegmentierung für Herzüberwachungs- und Herzrhythmusmanagementgeräte
|
Eigenschaften |
Wichtige Markteinblicke zu Geräten zur Herzüberwachung und Herzrhythmuskontrolle |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für Geräte zur Herzüberwachung und Herzrhythmuskontrolle in Nordamerika
Verbesserter Komfort durch fortschrittliche Herzüberwachungstechnologien
- Ein bedeutender und sich beschleunigender Trend auf dem US-Markt für Herzüberwachungs- und Herzrhythmusmanagementgeräte ist die zunehmende Integration fortschrittlicher Überwachungssysteme, einschließlich tragbarer und implantierbarer Geräte, in digitale Gesundheitsplattformen. Diese Integration verbessert den Patientenkomfort, die kontinuierliche Überwachung und die klinischen Erkenntnisse in Echtzeit erheblich.
- Moderne implantierbare Kardioverter-Defibrillatoren (ICDs) und Herzschrittmacher sind beispielsweise mittlerweile mit Fernüberwachungsfunktionen ausgestattet. So können Ärzte den Herzrhythmus ihrer Patienten in Echtzeit verfolgen, Anomalien frühzeitig erkennen und Behandlungspläne umgehend anpassen. Tragbare Herzmonitore ermöglichen eine nicht-invasive kontinuierliche Überwachung von Herzfrequenz und Herzrhythmus und bieten Patienten so mehr Kontrolle über ihr Gesundheitsmanagement.
- Moderne Herzüberwachungsgeräte verfügen zunehmend über Funktionen, die automatische Warnmeldungen bei Herzrhythmusstörungen, personalisierte Gesundheitsbenachrichtigungen und detaillierte Aktivitätsprotokolle ermöglichen. Diese Funktionen ermöglichen proaktive Interventionen, reduzieren Krankenhausaufenthalte und verbessern die allgemeine Patientenversorgung.
- Die nahtlose Integration von Herzüberwachungsgeräten in mobile und Cloud-basierte Gesundheitsplattformen ermöglicht die zentrale Überwachung mehrerer Gesundheitsparameter. Über eine einzige Schnittstelle können Ärzte und Patienten auf Herzrhythmusdaten, Medikamenteneinnahmeprotokolle und historische Trends zugreifen und so einen umfassenden und koordinierten Behandlungsansatz ermöglichen.
- Dieser Trend zu vernetzteren, zuverlässigeren und benutzerfreundlicheren Herzüberwachungssystemen verändert die Erwartungen der Patienten an die Herzversorgung grundlegend. Unternehmen wie Medtronic und Boston Scientific entwickeln daher Lösungen mit Echtzeit-Fernüberwachung, automatisierten Warnmeldungen und Interoperabilität mit elektronischen Patientenakten.
- Die Nachfrage nach fortschrittlichen Geräten zur Herzüberwachung und Rhythmuskontrolle steigt in Krankenhäusern, Fachkliniken und der häuslichen Pflege rasant an, da Patienten und Ärzte zunehmend Wert auf Komfort, Genauigkeit und kontinuierliches Pflegemanagement legen.
Marktdynamik für Geräte zur Herzüberwachung und Herzrhythmuskontrolle in Nordamerika
Treiber
Wachsender Bedarf aufgrund der zunehmenden Verbreitung von Herz-Kreislauf-Erkrankungen und der Einführung von Fernüberwachung
- Die zunehmende Verbreitung von Herz-Kreislauf-Erkrankungen wie Herzrhythmusstörungen, Herzinsuffizienz und anderen chronischen Erkrankungen sowie die zunehmende Verbreitung von Lösungen zur Fernüberwachung von Patienten sind ein wesentlicher Treiber für die erhöhte Nachfrage nach Geräten zur Herzüberwachung und zum Herzrhythmusmanagement.
- So gab PROCEPT BioRobotics im April 2024 die FDA-Zulassung seines Hydros-Robotersystems der nächsten Generation für die Aquablation-Therapie bekannt. Dies spiegelt die anhaltenden Innovationen bei minimalinvasiven kardiologischen und urologischen Eingriffen wider. Solche Strategien wichtiger Unternehmen dürften das Wachstum der Branche für Herzüberwachungs- und Herzrhythmusmanagementgeräte im Prognosezeitraum vorantreiben.
- Da sich Gesundheitsdienstleister und Patienten der Bedeutung einer kontinuierlichen Herzüberwachung immer mehr bewusst werden, ermöglichen fortschrittliche Geräte wie implantierbare Loop-Recorder, tragbare EKG-Monitore und mobile Herztelemetriesysteme die rechtzeitige Erkennung von Arrhythmien, erleichtern frühzeitige Interventionen und reduzieren die Zahl der erneuten Krankenhauseinweisungen.
- Darüber hinaus ermöglicht die Integration dieser Geräte mit Telemedizin-Plattformen und Fernüberwachungsanwendungen eine Datenübertragung in Echtzeit, sodass Kardiologen die Herzgesundheit der Patienten aus der Ferne überwachen und die Behandlung proaktiv anpassen können.
- Der Komfort minimalinvasiver Eingriffe, die kontinuierliche Überwachung, Echtzeit-Warnmeldungen und die Möglichkeit, Herzdaten mit Gesundheitsdienstleistern zu teilen, sind Schlüsselfaktoren für die Einführung dieser Geräte in Krankenhäusern, der häuslichen Pflege und ambulanten Pflegezentren. Der Trend zu vernetzten Gesundheitslösungen und patientenzentrierten Überwachungsgeräten trägt zusätzlich zum Marktwachstum bei.
Einschränkung/Herausforderung
Bedenken hinsichtlich der Gerätekomplexität und der hohen Anschaffungskosten
- Bedenken hinsichtlich der Komplexität bestimmter implantierbarer und Überwachungsgeräte stellen eine Herausforderung für eine breitere Marktakzeptanz dar. Fortgeschrittene Herzgeräte erfordern oft eine spezielle Schulung für die Implantation oder Programmierung, was die Zugänglichkeit in kleineren Gesundheitseinrichtungen einschränken kann.
- Beispielsweise können die hohen Anschaffungskosten von Geräten wie implantierbaren Loop-Recordern, Systemen zur kardialen Resynchronisationstherapie (CRT) und modernen Defibrillatoren für Krankenhäuser oder Patienten mit begrenztem Budget ein Hindernis darstellen.
- Für eine breitere Akzeptanz ist es entscheidend, diese Herausforderungen durch benutzerfreundliche Gerätedesigns, optimierte Implantationsverfahren, umfassende Schulungsprogramme für Kliniker und die Entwicklung kostengünstigerer Lösungen zu bewältigen. Darüber hinaus sind die Gewährleistung der Gerätezuverlässigkeit, der langfristigen Überwachungsgenauigkeit und der Patienten-Compliance entscheidende Faktoren für die Aufrechterhaltung des Vertrauens und der Wirksamkeit.
- Während die Kosten allmählich sinken und die Möglichkeiten der Fernüberwachung verbessert werden, können die wahrgenommene Komplexität und der anfängliche Investitionsbedarf immer noch eine breite Akzeptanz verhindern, insbesondere in kleineren Kliniken oder Entwicklungsregionen.
- Die Bewältigung dieser Herausforderungen durch vereinfachte Geräteschnittstellen, die Integration mit Telemedizin-Plattformen, Unterstützung bei der Versicherungsdeckung und Schulungsprogramme für Ärzte und Patienten wird für ein nachhaltiges Wachstum im Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle von entscheidender Bedeutung sein.
Marktumfang für Geräte zur Herzüberwachung und Herzrhythmuskontrolle in Nordamerika
Der Markt ist nach Produkt und Endbenutzer segmentiert.
- Nach Produkt
Der Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle ist produktbezogen in EKG-Geräte, implantierbare Loop-Recorder, Geräte zur Überwachung des Herzzeitvolumens (COM), Ereignismonitore, mobile kardiale Telemetrieüberwachung (MCT/MCOT), intelligente tragbare EKG-Monitore, Defibrillatoren, Herzschrittmacher und Geräte zur kardialen Resynchronisationstherapie (CRT) unterteilt. Das Segment der Herzschrittmacher hatte im Jahr 2024 mit 32,5 % den größten Marktanteil, was auf ihre weit verbreitete klinische Anwendung zur Behandlung von Bradykardie und anderen Herzrhythmusstörungen zurückzuführen ist. Herzschrittmacher werden aufgrund ihrer nachgewiesenen Wirksamkeit, Zuverlässigkeit und kontinuierlichen technologischen Innovationen wie frequenzadaptiver Stimulation und MRT-Kompatibilität stark bevorzugt. Die starke Akzeptanz in Krankenhäusern und Fachkliniken, kombiniert mit starken Erstattungsrahmen und einer wachsenden älteren Bevölkerung in Nordamerika, stärkt ihre führende Position auf dem Markt. Kontinuierliche Produkteinführungen und Verbesserungen der Gerätelebensdauer stärken die Dominanz des Herzschrittmachersegments weiter.
Das Segment der intelligenten tragbaren EKG-Monitore wird voraussichtlich die schnellste Wachstumsrate verzeichnen und von 2025 bis 2032 eine durchschnittliche jährliche Wachstumsrate (CAGR) von 15,8 % verzeichnen. Dies ist auf das steigende Verbraucherbewusstsein für Herz-Kreislauf-Gesundheit und die zunehmende Nutzung von Heimüberwachungslösungen zurückzuführen. Diese Geräte ermöglichen eine Echtzeitüberwachung von Herzfrequenz und Herzrhythmus und lassen sich nahtlos in mobile Apps integrieren, sodass Ärzte sie aus der Ferne überwachen können. Ihre Tragbarkeit, ihr nicht-invasives Design und ihre Benutzerfreundlichkeit machen sie ideal für ambulante Patienten und Personen mit Herzrhythmusstörungen. Die zunehmende Präferenz für präventive Herzbehandlungen, kombiniert mit technologischen Innovationen wie KI-gestützter Anomalieerkennung und Cloud-Datenanalyse, treibt die schnelle Akzeptanz sowohl im klinischen als auch im häuslichen Umfeld voran.
- Nach Endbenutzer
Der Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle ist nach Endnutzern in Krankenhäuser, ambulante und häusliche Pflege und andere Bereiche unterteilt. Das Segment Krankenhäuser hatte im Jahr 2024 mit 58,3 % den größten Marktanteil, was auf die hohe Zahl an Herzpatienten zurückzuführen ist, die eine kontinuierliche Überwachung, die Implantation hochentwickelter Geräte und die postoperative Versorgung benötigen. Krankenhäuser bieten spezialisierte Infrastruktur, geschultes medizinisches Fachpersonal und fortschrittliche Diagnosemöglichkeiten, was die flächendeckende Einführung komplexer Geräte wie Herzschrittmacher, CRT-Geräte und Defibrillatoren ermöglicht. Gut etablierte kardiologische Abteilungen, strenge Erstattungsrichtlinien und regelmäßige Geräte-Upgrades tragen zur anhaltenden Umsatzdominanz in diesem Segment bei. Krankenhäuser profitieren zudem von groß angelegten Beschaffungsaufträgen, was ihre Marktführerschaft weiter stärkt.
Im Bereich der häuslichen und ambulanten Pflege wird von 2025 bis 2032 mit 14,6 % die höchste durchschnittliche jährliche Wachstumsrate erwartet. Grund hierfür ist die zunehmende Nachfrage nach Lösungen zur Fernüberwachung des Herzens und zur ambulanten Versorgung. Da die Reduzierung von Krankenhauswiederaufnahmen zunehmend im Fokus steht, gewinnen tragbare und mobile Herzüberwachungsgeräte bei Patienten und Gesundheitsdienstleistern an Bedeutung. Diese Geräte ermöglichen eine kontinuierliche Überwachung von Herzrhythmusstörungen, die frühzeitige Erkennung von Anomalien und ein rechtzeitiges Eingreifen des Arztes. Dies sorgt für Komfort und eine verbesserte Versorgungsqualität. Technologische Fortschritte sowie das steigende Bewusstsein der Verbraucher für Selbstüberwachung und präventive Gesundheitsfürsorge beschleunigen das Wachstum in diesem Segment.
Regionale Analyse des nordamerikanischen Marktes für Herzüberwachungs- und Herzrhythmusmanagementgeräte
- Nordamerika dominierte den Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle mit dem größten Umsatzanteil im Jahr 2024
- Angetrieben durch eine fortschrittliche Gesundheitsinfrastruktur, eine hohe Prävalenz von Herzerkrankungen und die zunehmende Verbreitung innovativer Geräte wie implantierbare Kardioverter-Defibrillatoren (ICDs), Herzschrittmacher und tragbare Herzmonitore
- Der Markt in der Region wird zusätzlich durch häufige Produkteinführungen, kontinuierliche technologische Fortschritte und solide Erstattungsrahmen unterstützt, die einen breiten Zugang der Patienten zu modernen Lösungen für die Herzversorgung gewährleisten.
Markteinblicke für Herzüberwachungs- und Herzrhythmusmanagementgeräte in den USA
Der US-Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle erzielte 2024 mit 74,8 % den größten Umsatzanteil in Nordamerika, angetrieben durch die starke Verbreitung fortschrittlicher Technologien zur Herzüberwachung und Herzrhythmuskontrolle. Wichtige Wachstumstreiber sind das hohe Bewusstsein für Herz-Kreislauf-Erkrankungen, ein technologisch fortschrittliches Gesundheitssystem und die zunehmende Nutzung implantierbarer und tragbarer Herzgeräte zur kontinuierlichen Patientenüberwachung. Die regelmäßige Einführung innovativer Geräte, darunter Herzschrittmacher der nächsten Generation, ICDs, CRT-Systeme und intelligente tragbare EKG-Monitore, sowie eine starke Kostenerstattungspolitik treiben das Marktwachstum weiter voran. Die Integration von Telemedizin- und Fernüberwachungsplattformen ermöglicht eine Echtzeit-Patientenverfolgung, unterstützt frühzeitige Interventionen, eine verbesserte Therapietreue und verbesserte klinische Ergebnisse und stärkt so die Marktdominanz der USA.
Markteinblick in Kanada für Geräte zur Herzüberwachung und Herzrhythmuskontrolle
Der kanadische Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle wird im Prognosezeitraum voraussichtlich das am schnellsten wachsende Land Nordamerikas sein und eine durchschnittliche jährliche Wachstumsrate (CAGR) von 7,8 % verzeichnen . Diese Entwicklung wird durch staatliche Initiativen zur Stärkung der Herzversorgung, zur Verbesserung des Zugangs zu Krankenhäusern und Fachkliniken sowie zur Förderung der präventiven Kardiologie vorangetrieben. Die Einführung tragbarer Herzmonitore und Telemonitoring-Technologien der nächsten Generation beschleunigt das Wachstum zusätzlich und ermöglicht eine kontinuierliche Patientenüberwachung, die Früherkennung von Herzrhythmusstörungen und rechtzeitige klinische Interventionen. Das wachsende Bewusstsein von Patienten und Gesundheitsdienstleistern für die Fernversorgung von Herzpatienten sowie steigende Investitionen in digitale Gesundheitslösungen dürften das starke Marktwachstum Kanadas im gesamten Prognosezeitraum aufrechterhalten.
Marktanteil von Geräten zur Herzüberwachung und Herzrhythmuskontrolle in Nordamerika
Die Branche der Geräte zur Herzüberwachung und Herzrhythmuskontrolle wird hauptsächlich von etablierten Unternehmen angeführt, darunter:
- GENERAL ELECTRIC COMPANY (USA)
- Abbott (USA)
- Boston Scientific Corporation (USA)
- SCHILLER AG (Schweiz)
- Koninklijke Philips NV (Niederlande)
- Biotronik SE & Co., KG (Deutschland)
- ABIOMED (USA)
- NIHON KOHDEN CORPORATION (Japan)
- ReliantHeart Inc. (USA)
- Berlin Heart (Deutschland)
- Jarvik Heart, Inc. (USA)
- LivaNova PLC (Großbritannien)
- ZOLL Medical Corporation (USA)
- Edwards Lifesciences Corporation (USA)
- Getinge AB (Schweden)
Neueste Entwicklungen auf dem nordamerikanischen Markt für Geräte zur Herzüberwachung und Herzrhythmuskontrolle
- Im Dezember 2024 gab HeartBeam, Inc., ein Medizintechnikunternehmen, das sich der Verbesserung der Herzmedizin durch innovative Diagnoseinstrumente verschrieben hat, bekannt, dass es die 510(k)-Zulassung der US-amerikanischen Food and Drug Administration (FDA) für sein HeartBeam-System erhalten hat. Dieses Gerät bietet umfassende Arrhythmie-Erkennung mit einem einzigartigen, patentierten Ansatz und verfügt über eine hochauflösende EKG-Funktion in einem kompakten, kreditkartengroßen, kabellosen Design. Das HeartBeam-System ermöglicht die Erfassung der Herzaktivität aus drei verschiedenen Blickwinkeln und liefert so wichtige Erkenntnisse zur Herzgesundheit.
- Im Mai 2024 gab die Heart Rhythm Society (HRS) neue Ergebnisse mehrerer klinischer Studien bekannt, die die jüngsten Fortschritte in der Technologie und Pharmakotherapie implantierbarer elektronischer Herzgeräte (CIED) aufzeigten. Diese Studien, die im Rahmen der Heart Rhythm 2024 als bahnbrechende klinische Wissenschaft präsentiert wurden, zeigten Entwicklungen auf, die die Grenzen herkömmlicher Geräte überwinden oder ungedeckten therapeutischen Bedarf decken.
- Im April 2024 brachte Abbott den weltweit ersten Smartphone-kompatiblen implantierbaren Herzmonitor auf den Markt, den Confirm Rx ICM. Dieses Gerät ermöglicht eine präzisere Erkennung von Herzrhythmusstörungen und bietet Patienten eine intelligentere 24/7-Herzüberwachung. Der Confirm Rx ICM ist für die nahtlose Integration mit Smartphones konzipiert und ermöglicht Datenaustausch in Echtzeit sowie eine verbesserte Patienteneinbindung.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.