Nordamerikanischer Markt für Anästhesie- und Beatmungsgeräte, nach Produkt (Anästhesiegeräte, Beatmungsgeräte), Endbenutzer (Krankenhäuser, Kliniken, häusliche Pflege, ambulante Servicezentren), Land (USA, Kanada, Mexiko), Branchentrends und Prognose bis 2029.
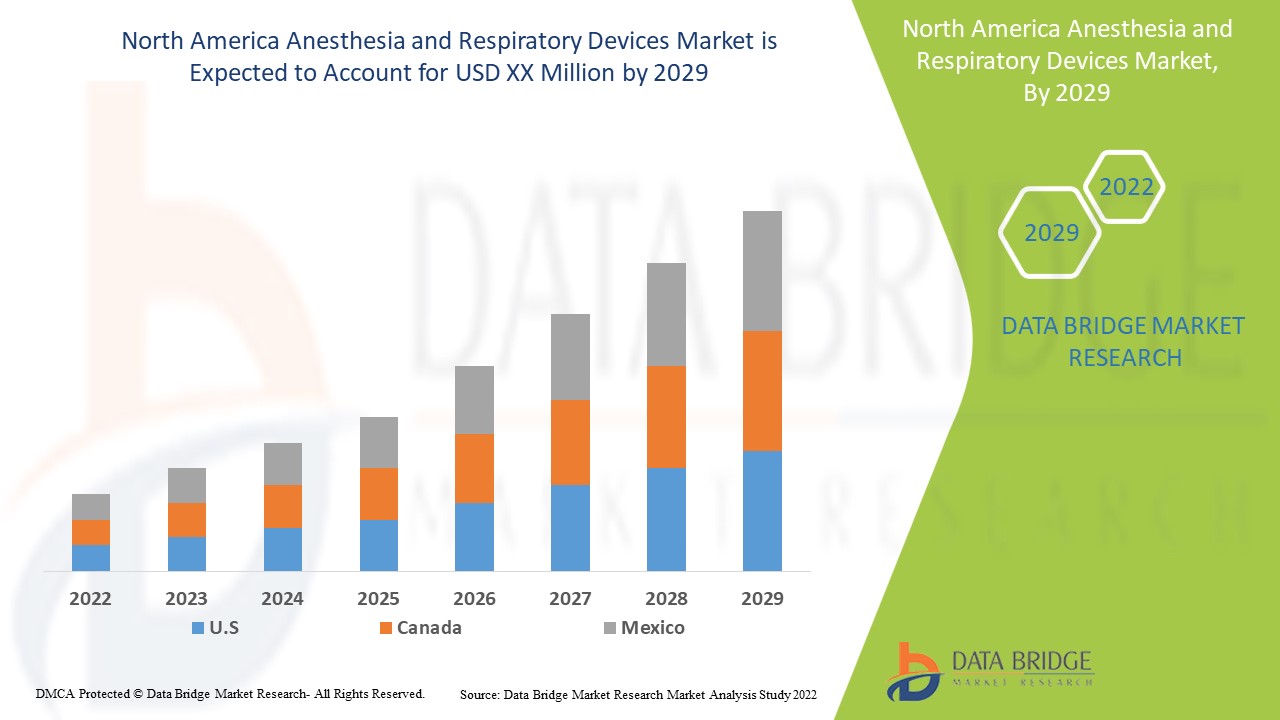 Marktanalyse und Einblicke in den Markt für Anästhesie- und Beatmungsgeräte
Marktanalyse und Einblicke in den Markt für Anästhesie- und Beatmungsgeräte
Es wird erwartet, dass der Markt für Anästhesie- und Beatmungsgeräte im Prognosezeitraum von 2022 bis 2029 an Marktwachstum gewinnt. Data Bridge Market Research geht davon aus, dass der Markt im oben genannten Prognosezeitraum mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 7,75 % wachsen wird.
Unter Anästhesie- und Beatmungsgeräten versteht man Geräte, die bei chirurgischen Eingriffen grundsätzlich eine kontinuierliche Versorgung mit medizinischen Gasen wie Sauerstoff und Lachgas gewährleisten, um Schmerzen, Blutdruck, Herzfrequenz, Atmung und Blutfluss zu kontrollieren. Der Schwerpunkt der Beatmungsgeräte liegt auf der Behandlung, Bewertung, Diagnose, Kontrolle, Betreuung und Pflege von Patienten, die an akuten und chronischen Atemwegserkrankungen leiden.
Der Anstieg der Zahl geriatrischer Menschen und die Zunahme von Atemwegserkrankungen sind die wesentlichen Faktoren, die das Wachstum des Marktes für Anästhesie- und Beatmungsgeräte vorantreiben. Darüber hinaus steigern der Anstieg der Zahl älterer Menschen in dieser Region, die zunehmende Häufigkeit von Atemwegserkrankungen und der technologische Fortschritt bei der Anästhesieüberwachung das Gesamtwachstum des Marktes. Allerdings dürften die durch Beatmungsgeräte verursachten Schäden an Frühgeborenen und die höheren Kosten der Geräte das Wachstum des Marktes behindern.
Die technologischen Fortschritte in der Anästhesieüberwachung dürften lukrative Chancen für den Markt schaffen. Auf der anderen Seite könnte das mangelnde Bewusstsein das Marktwachstum gefährden.
Dieser Marktbericht für Anästhesie- und Beatmungsgeräte enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-/Exportanalysen, Produktionsanalysen, Optimierung der Wertschöpfungskette, Marktanteilen, Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neue Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um weitere Informationen zum Markt für Anästhesie- und Beatmungsgeräte von Data Bridge Market Research zu erhalten, kontaktieren Sie uns für ein Analyst Briefing. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Marktumfang und Marktgröße für Anästhesie- und Beatmungsgeräte in Nordamerika
Der Markt für Anästhesie- und Beatmungsgeräte ist nach Produkt und Endverbraucher segmentiert. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern wertvolle Marktübersichten und Markteinblicke, die ihnen bei der strategischen Entscheidungsfindung zur Identifizierung der wichtigsten Marktanwendungen helfen.
- Basierend auf dem Produkttyp ist der Markt in Anästhesiegeräte und Beatmungsgeräte unterteilt. Der Markt für Anästhesiegeräte ist weiter unterteilt in Anästhesiegeräte, Anästhesie-Verbrauchsmaterial und Zubehör, Anästhesiemonitore, Anästhesie-Informationsmanagementsysteme (AIMS). Anschließend werden Anästhesie-Verbrauchsmaterial und Zubehör weiter unterteilt in Anästhesiekreisläufe, Anästhesiemasken, Beatmungsgeräte, Laryngoskope, flexible Intubationsendoskope, supraglottische Atemwege, Beatmungskreisläufe, HMEs, Regionalanästhesie- und Schmerzgeräte, Sonstiges und Anästhesiemonitore werden weiter unterteilt in einfache Anästhesiemonitore, fortschrittliche Anästhesiemonitore, integrierte Anästhesiearbeitsplätze. Der Markt für Beatmungsgeräte wird weiter unterteilt in Therapiegeräte, Masken, Beatmungsgeräte, Vernebler, Luftbefeuchter, Sauerstoffkonzentratoren, Inhalatoren, wiederverwendbare Beatmungsgeräte, Beatmungsgeräte für Erwachsene, Beatmungsgeräte für Säuglinge/Neugeborene, Stickoxid-Abgabeeinheiten, Kapnographen, Gasanalysatoren, Sauerstoffhauben. Anschließend werden Therapiegeräte weiter unterteilt in Geräte mit positivem Atemwegsdruck (PAP) und Geräte mit positivem Atemwegsdruck (PAP) weiter unterteilt in Geräte mit kontinuierlichem positivem Atemwegsdruck (CPAP), Geräte mit automatischem positivem Atemwegsdruck (APAP) und Geräte mit zweistufigem positivem Atemwegsdruck (BPAP). Masken werden weiter unterteilt in Nasenmasken, Vollmasken, Nasenmasken und Mundmasken. Beatmungsgeräte werden weiter unterteilt in Beatmungsgeräte für Erwachsene und Beatmungsgeräte für Säuglinge/Neugeborene. Sauerstoffkonzentratoren werden weiter unterteilt in feste Sauerstoffkonzentratoren und feste Sauerstoffkonzentratoren werden weiter unterteilt in tragbare Sauerstoffkonzentratoren. Inhalatoren werden weiter unterteilt in Trockenpulverinhalatoren, Dosieraerosole und Soft-Mist-Inhalatoren. Wiederverwendbare Beatmungsgeräte werden weiter unterteilt in Beatmungsgeräte für Erwachsene und Beatmungsgeräte für Säuglinge/Neugeborene. Das Segment Überwachungsgeräte wird nach Produkttyp weiter unterteilt in Pulsoximeter, anschließend werden Pulsoximeter weiter unterteilt in Pädiatrie-Pulsoximeter, am Handgelenk getragene Pulsoximeter, Handoximeter, Fingerspitzen-Pulsoximeter, Tisch- oder Nachttisch-Pulsoximeter. Das Segment Diagnosegeräte wird nach Produkttyp weiter unterteilt in Spirometer , Polysomnographiegeräte (PSG), anschließend werden Polysomnographiegeräte (PSG) weiter unterteilt in Peak-Flow-Meter. Das Segment Verbrauchsmaterialien und Zubehör wird nach Produkttyp weiter unterteilt in Einweg-Beatmungsgeräte, Tracheostomietuben, Nasenkanülen, Einwegmasken und Sonstiges.
- Auf der Grundlage des Endbenutzers ist der Markt für Anästhesie- und Beatmungsgeräte in Krankenhäuser, Kliniken, Einrichtungen der häuslichen Pflege und ambulante Servicezentren segmentiert.
Anästhesie- und Beatmungsgeräte Markt – Länderebene Analyse
Der Markt für Anästhesie- und Beatmungsgeräte wird analysiert und Einblicke in die Marktgröße und Trends werden wie oben angegeben nach Produkt und Endbenutzer bereitgestellt.
Die im Marktbericht für Anästhesie- und Beatmungsgeräte abgedeckten Länder sind die USA, Kanada und Mexiko in Nordamerika.
Der Länderabschnitt des Marktberichts für Anästhesie- und Beatmungsgeräte enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Regulierung auf dem Inlandsmarkt, die sich auf die aktuellen und zukünftigen Trends des Marktes auswirken. Datenpunkte wie Verbrauchsmengen, Produktionsstandorte und -mengen, Import-Export-Analyse, Preistrendanalyse, Rohstoffkosten, Downstream- und Upstream-Wertschöpfungskettenanalyse sind einige der wichtigsten Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Bei der Prognoseanalyse der Länderdaten werden auch die Präsenz und Verfügbarkeit nordamerikanischer Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen inländischer Zölle und Handelsrouten berücksichtigt.
Wachstum der Gesundheitsinfrastruktur, installierte Basis und Durchdringung mit neuen Technologien
Der Markt für Anästhesie- und Beatmungsgeräte bietet Ihnen außerdem eine detaillierte Marktanalyse für jedes Land, das Wachstum der Gesundheitsausgaben für Investitionsgüter, die installierte Basis verschiedener Arten von Produkten für den Markt für Anästhesie- und Beatmungsgeräte, die Auswirkungen der Technologie anhand von Lebenslinienkurven und Änderungen der regulatorischen Szenarien im Gesundheitswesen und deren Auswirkungen auf den Markt für Anästhesie- und Beatmungsgeräte. Die Daten sind für den historischen Zeitraum 2010 bis 2020 verfügbar.
Wettbewerbsumfeld und Anästhesie- und Beatmungsgeräte Marktanteilsanalyse
Die Wettbewerbslandschaft auf dem Markt für Anästhesie- und Beatmungsgeräte liefert Einzelheiten nach Wettbewerbern. Die enthaltenen Einzelheiten umfassen Unternehmensübersicht, Unternehmensfinanzen, erzielten Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, Präsenz in Nordamerika, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang, Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen in Bezug auf den Markt für Anästhesie- und Beatmungsgeräte.
Zu den wichtigsten Akteuren auf dem Markt für Anästhesie- und Beatmungsgeräte zählen unter anderem Baxter, BD, B. Braun Melsungen AG, Pfizer Inc., Ambu A/S., Cardinal Health, Smith+Nephew, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., PerkinElmer Inc., Medline Industries, Inc., Masimo., KCWW, GENERAL ELECTRIC, Koninklijke Philips NV, SCHILLER, Drägerwerk AG & Co. KGaA, NIHON KOHDEN CORPORATION, Medtronic und Westmed, Inc.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.














