Middle East And Africa Multiple Hereditary Exostosis Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
4.80 Million
USD
6.51 Million
2024
2032
USD
4.80 Million
USD
6.51 Million
2024
2032
| 2025 –2032 | |
| USD 4.80 Million | |
| USD 6.51 Million | |
|
|
|
|
Marktsegmentierung für multiple hereditäre Exostosen im Nahen Osten und in Afrika nach Typ (sitzend und gestielt), Behandlung (Operation, Medikamente und andere), Diagnose (Röntgen, Computertomographie (CT), Magnetresonanztomographie (MRT), genetische Tests und andere), Stelle (Beine, Arme, Schultern, Becken, Finger und Zehen), Altersgruppe (Kinder und Erwachsene), Endbenutzer (Krankenhäuser, Fachkliniken, ambulante chirurgische Zentren und andere) – Branchentrends und Prognose bis 2032
Marktgröße für multiple hereditäre Exostosen im Nahen Osten und Afrika
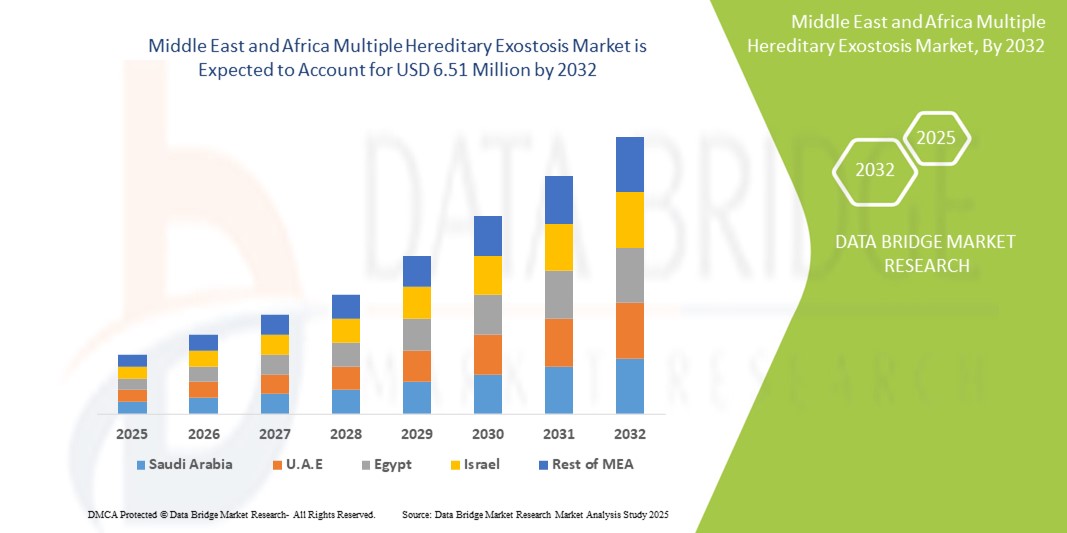
- Der Markt für multiple hereditäre Exostosen im Nahen Osten und Afrika wurde im Jahr 2024 auf 4,80 Millionen US-Dollar geschätzt und dürfte bis 2032 6,51 Millionen US-Dollar erreichen , bei einer CAGR von 3,90 % im Prognosezeitraum.
- Das Marktwachstum wird maßgeblich durch das gestiegene Bewusstsein für seltene genetische Erkrankungen, die Verbesserung der diagnostischen Infrastruktur und die schrittweise Weiterentwicklung genetischer Testtechnologien in der gesamten Region vorangetrieben.
- Darüber hinaus schaffen die zunehmende Unterstützung der Forschung zu seltenen Krankheiten durch staatliche und gemeinnützige Organisationen sowie steigende Investitionen in personalisierte und Präzisionsmedizin ein günstigeres Umfeld für die Frühdiagnose und -behandlung von MHE. Diese zusammenlaufenden Faktoren tragen zu einer stetigen Akzeptanz zielgerichteter Therapien und genetischer Dienstleistungen bei und treiben so das Marktwachstum in der gesamten MEA-Region voran.
Marktanalyse für multiple hereditäre Exostose im Nahen Osten und Afrika
- Die Multiple Hereditäre Exostose (MHE), eine seltene genetische Erkrankung, die durch das Wachstum mehrerer gutartiger Knochentumoren gekennzeichnet ist, erfährt im Nahen Osten und Afrika zunehmende Aufmerksamkeit aufgrund verbesserter Diagnosemöglichkeiten, wachsender Patientenvertretung und eines zunehmenden Fokus auf die Gesundheitsversorgung seltener Krankheiten in der gesamten Region.
- Die Nachfrage nach Frühdiagnose und fortschrittlichen Behandlungsmöglichkeiten für MHE wird durch das zunehmende Bewusstsein bei Gesundheitsdienstleistern und Patienten sowie den verbesserten Zugang zu genetischer Beratung und Bildgebungstechnologien vorangetrieben.
- Südafrika dominierte den Markt für multiple hereditäre Exostosen im Nahen Osten und Afrika mit dem größten Umsatzanteil von 28,9 % im Jahr 2024, unterstützt durch seine relativ fortschrittliche Gesundheitsinfrastruktur, aktive Forschungsprogramme zu genetischen Störungen und das Vorhandensein spezialisierter medizinischer Einrichtungen, die sich auf seltene Erkrankungen spezialisiert haben.
- Die Vereinigten Arabischen Emirate (VAE) werden im Prognosezeitraum voraussichtlich das am schnellsten wachsende Land im Markt für multiple hereditäre Exostose im Nahen Osten und Afrika sein, aufgrund steigender staatlicher Gesundheitsausgaben, eines zunehmenden Medizintourismus und der Einführung von Registern für seltene Krankheiten und Initiativen zur Präzisionsmedizin.
- Das sessile Segment dominierte den Markt für multiple hereditäre Exostosen im Nahen Osten und Afrika mit einem Marktanteil von 55,2 % im Jahr 2024, was auf die höhere Prävalenz bei Patienten und die einfachere Erkennung durch Standardbildgebungsverfahren zurückzuführen ist
Berichtsumfang und Marktsegmentierung für multiple hereditäre Exostose im Nahen Osten und Afrika
|
Eigenschaften |
Wichtige Markteinblicke in den Nahen Osten und Afrika im Bereich der multiplen hereditären Exostose |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Naher Osten und Afrika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für multiple hereditäre Exostosen im Nahen Osten und Afrika
„Fortschritte bei genetischen Tests und Frühdiagnosen“
- Ein bedeutender und sich beschleunigender Trend auf dem Markt für multiple hereditäre Exostose (MHE) im Nahen Osten und Afrika ist die zunehmende Nutzung fortschrittlicher genetischer Test- und Bildgebungstechnologien, die die Frühdiagnose und personalisierte Pflegestrategien für betroffene Personen in der gesamten Region verbessern.
- So integrieren führende Gesundheitseinrichtungen in Ländern wie den Vereinigten Arabischen Emiraten und Südafrika zunehmend Next-Generation-Sequencing (NGS) und MRI-basierte Überwachung in die Standardbehandlung seltener Skeletterkrankungen wie MHE.
- Genetische Tests ermöglichen die frühzeitige Identifizierung der für MHE verantwortlichen EXT1- und EXT2-Genmutationen und ermöglichen so rechtzeitige Interventionen und verbesserte klinische Ergebnisse. Institutionen in Saudi-Arabien und Ägypten arbeiten bereits mit internationalen Genlaboren zusammen, um den Zugang zu diesen Diagnoseinstrumenten zu erweitern.
- Die Verfügbarkeit von MRT- und CT-Scans in Zentren der tertiären Versorgung ermöglicht eine genaue Kartierung des Tumorwachstums und von Skelettdeformationen und unterstützt so eine bessere Operationsplanung und eine langfristige Überwachung.
- Diese Fortschritte in der Diagnostik tragen zu einem Wandel hin zu einem proaktiveren Krankheitsmanagement bei und ermöglichen es den Gesundheitsdienstleistern, individuelle Behandlungspfade zu entwickeln und Funktionsbeeinträchtigungen zu verzögern oder zu verhindern.
- Da das Bewusstsein für MHE bei medizinischem Fachpersonal und Patienten wächst, wird die Nachfrage nach umfassenden diagnostischen Untersuchungen voraussichtlich steigen, was die Rolle der Präzisionsmedizin im Bereich der seltenen Krankheiten in der MEA-Region weiter stärkt.
Marktdynamik für multiple hereditäre Exostosen im Nahen Osten und Afrika
Treiber
„Wachsendes Bewusstsein, spezialisierte Versorgung und unterstützende Regierungspolitik“
- Das zunehmende Bewusstsein für seltene genetische Erkrankungen wie MHE, gepaart mit der Verbesserung der Gesundheitsinfrastruktur und der Verfügbarkeit spezialisierter Pflegezentren, treibt das Marktwachstum im Nahen Osten und in Afrika voran.
- So haben beispielsweise die staatlich finanzierten Programme für seltene Krankheiten und die Initiativen zur genetischen Beratung in Südafrika die Früherkennung und den Zugang zu Behandlungsmöglichkeiten für Patienten mit MHE deutlich verbessert.
- Die staatlichen Bemühungen in Ländern wie Saudi-Arabien und den Vereinigten Arabischen Emiraten, in Register für seltene Krankheiten zu investieren, die Genommedizin zu fördern und den Zugang zu orthopädischer Chirurgie und Physiotherapie zu verbessern, schaffen ein günstiges Umfeld für die Marktexpansion.
- Die zunehmende Präsenz von Interessengruppen und medizinischen Aufklärungsprogrammen trägt ebenfalls dazu bei, die Stigmatisierung zu verringern und die Patienten besser über die langfristigen Auswirkungen einer unbehandelten MHE aufzuklären.
- Der zunehmende Medizintourismus zu spezialisierten orthopädischen Zentren in der Golfregion ist ein weiterer Faktor, der zur steigenden Nachfrage nach chirurgischen Behandlungen und postoperativen Rehabilitationsdiensten beiträgt.
Einschränkung/Herausforderung
„Hohe Kosten für Gentests und geringes Bewusstsein in abgelegenen Gebieten“
- Trotz der Fortschritte schränken die hohen Kosten genetischer Tests und spezieller bildgebender Verfahren weiterhin den breiten Zugang zu einer frühen MHE-Diagnose ein, insbesondere in einkommensschwachen und ländlichen Gebieten.
- In vielen Ländern Subsahara-Afrikas gibt es noch immer erhebliche Lücken in der diagnostischen Infrastruktur. CT- oder MRT-Geräte sind nur begrenzt verfügbar, und es mangelt an ausgebildeten genetischen Beratern oder orthopädischen Spezialisten.
- Die Seltenheit der Erkrankung und das Fehlen nationaler Aufklärungskampagnen in einigen Regionen führen häufig zu verzögerten Diagnosen, Fehldiagnosen oder unzureichender Langzeitpflege, was zu einer Verschlechterung der Patientenergebnisse führt.
- Die Eigenbeteiligung für Operationen und Nachsorge kann erheblich sein, insbesondere in Systemen mit eingeschränktem Versicherungsschutz oder staatlicher Unterstützung für seltene Krankheiten
- Um diese Herausforderungen zu bewältigen, bedarf es sektorübergreifender Zusammenarbeit, einer verstärkten öffentlich-privaten Finanzierung von Programmen für seltene Krankheiten und einer erweiterten Ausbildung von medizinischem Fachpersonal in der Identifizierung und Behandlung genetisch bedingter Skeletterkrankungen wie MHE.
Marktumfang für multiple hereditäre Exostosen im Nahen Osten und Afrika
Der Markt ist nach Typ, Behandlung, Diagnose, Standort, Altersgruppe und Endbenutzer segmentiert.
- Nach Typ
Der Markt für multiple hereditäre Exostosen im Nahen Osten und Afrika ist nach Typ in sessile und gestielte Exostosen unterteilt. Das sessile Segment dominierte den Markt mit dem größten Umsatzanteil von 55,2 % im Jahr 2024, was auf die höhere Prävalenz bei MHE-Fällen und die einfachere Identifizierung durch bildgebende Verfahren wie Röntgen und MRT zurückzuführen ist. Sessile Osteochondrome erfordern aufgrund ihrer breiteren Basis und des höheren Komplikationspotenzials wie Nervenkompression oder -deformität oft eine engmaschigere Überwachung und Intervention. Dies hat den Bedarf an Diagnostik und Behandlung für diesen Subtyp erhöht.
Das Segment der gestielten Knochen dürfte zwischen 2025 und 2032 die höchste durchschnittliche jährliche Wachstumsrate aufweisen, da die verbesserte Zugänglichkeit von Bildgebungstechniken eine bessere Identifizierung und Differenzierung von Knochenläsionsarten ermöglicht, insbesondere in Fachkliniken und städtischen Krankenhäusern.
- Nach Behandlung
Der Markt für multiple hereditäre Exostosen im Nahen Osten und Afrika ist hinsichtlich der Behandlung in Chirurgie, Medikamente und andere Bereiche unterteilt. Das Segment Chirurgie hatte 2024 den größten Marktanteil, da die chirurgische Entfernung nach wie vor die primäre Behandlungsmethode für symptomatische Osteochondrome ist, insbesondere für solche, die Schmerzen, Deformitäten oder Funktionseinschränkungen verursachen. Die zunehmende orthopädische Spezialisierung in regionalen Krankenhäusern und der Medizintourismus in Länder wie die Vereinigten Arabischen Emirate und Südafrika unterstützen dieses Segment ebenfalls.
Das Medikamentensegment wird voraussichtlich von 2025 bis 2032 die höchste jährliche Wachstumsrate verzeichnen. Es wird hauptsächlich zur Schmerzbehandlung und Entzündungskontrolle eingesetzt, hat aber nach wie vor eher unterstützende als heilende Wirkung. Innovationen in der genspezifischen Therapie und der unterstützenden pharmazeutischen Versorgung könnten zum langfristigen Wachstum in diesem Segment beitragen.
- Nach Diagnose
Der Markt für multiple hereditäre Exostose im Nahen Osten und Afrika ist auf Grundlage der Diagnose in Röntgen, Computertomographie (CT), Magnetresonanztomographie (MRT), genetische Tests und weitere Verfahren unterteilt. Das Röntgensegment ist aufgrund seiner breiten Verfügbarkeit und Kosteneffizienz bei der Erkennung von Knochenauswüchsen im Zusammenhang mit MHE marktführend. Es ist häufig das erste Diagnoseinstrument in öffentlichen und privaten Gesundheitseinrichtungen.
Die MRT wird voraussichtlich bis 2032 die höchste Wachstumsrate verzeichnen, da sie eine detailliertere Visualisierung von Knorpelkapseln und Weichteilbefall ermöglicht, die für die Operationsplanung entscheidend ist. Steigende Investitionen in fortschrittliche diagnostische Infrastruktur in städtischen Zentren Saudi-Arabiens, der VAE und Ägyptens unterstützen diesen Trend.
- Nach Site
Der Markt für multiple hereditäre Exostose im Nahen Osten und Afrika ist nach Standorten in Beine, Arme, Schultern, Becken, Finger und Zehen unterteilt. Das Beinsegment hatte 2024 den größten Anteil, da Deformitäten der unteren Extremitäten bei MHE-Patienten am häufigsten auftreten und oft zu Gangstörungen und orthopädischen Eingriffen führen. Der Fokus auf die Korrektur von Beinlängendifferenzen und Winkeldeformitäten hat die Nachfrage nach Frühdiagnosen und Korrekturoperationen erhöht.
Im Bereich Arme und Schultern wird von 2025 bis 2032 voraussichtlich die höchste durchschnittliche jährliche Wachstumsrate (CAGR) zu verzeichnen sein, insbesondere bei pädiatrischen Patienten, bei denen wachstumsbedingte Komplikationen häufig sind.
- Nach Altersgruppe
Der Markt für multiple hereditäre Exostose im Nahen Osten und Afrika ist nach Altersgruppen in Kinder und Erwachsene unterteilt. Das pädiatrische Segment dominierte den Markt im Jahr 2024, da MHE eine genetische Erkrankung ist, die sich häufig in der frühen Kindheit manifestiert und deren sichtbare Symptome vor der Pubertät auftreten. Kinder benötigen während ihrer Wachstumsphase häufig eine Langzeitüberwachung und mehrere Eingriffe, was zu einer höheren Nachfrage nach Diagnose- und Behandlungsleistungen in dieser Gruppe führt.
Im Erwachsenensegment wird von 2025 bis 2032 voraussichtlich die höchste durchschnittliche jährliche Wachstumsrate zu verzeichnen sein, was auf die zunehmende Diagnose spät auftretender oder zuvor nicht diagnostizierter Fälle sowie auf die kontinuierliche Überwachung von MHE-Überlebenden im Kindesalter zurückzuführen ist.
- Nach Endbenutzer
Der Markt für multiple hereditäre Exostose im Nahen Osten und Afrika ist nach Endverbrauchern in Krankenhäuser, Fachkliniken, ambulante chirurgische Zentren und weitere Bereiche unterteilt. Das Krankenhaussegment war 2024 mit dem größten Marktanteil führend, da die meisten chirurgischen Eingriffe und fortgeschrittenen Diagnosedienste in Krankenhäusern mit multidisziplinärer Expertise zentralisiert durchgeführt werden.
Fachkliniken werden im Prognosezeitraum voraussichtlich das schnellste Wachstum verzeichnen, unterstützt durch die steigende Zahl orthopädischer und genetischer Kliniken für seltene Erkrankungen in städtischen Ballungszentren in Saudi-Arabien, den Vereinigten Arabischen Emiraten und Südafrika. Diese Kliniken bieten spezialisierte, patientenorientierte Versorgung mit schnellerer Diagnose und Zugang zu chirurgischer Expertise.
Regionale Analyse des Marktes für multiple hereditäre Exostose im Nahen Osten und Afrika
- Südafrika dominierte den Markt für multiple hereditäre Exostosen im Nahen Osten und Afrika mit dem größten Umsatzanteil von 28,9 % im Jahr 2024, unterstützt durch seine relativ fortschrittliche Gesundheitsinfrastruktur, aktive Forschungsprogramme zu genetischen Störungen und das Vorhandensein spezialisierter medizinischer Einrichtungen, die sich auf seltene Erkrankungen spezialisiert haben.
- Das Gesundheitssystem des Landes legt Wert auf Frühdiagnose und personalisierte Betreuung. Der Zugang zu MRT, genetischen Tests und spezialisierter orthopädischer Behandlung wird zunehmend erweitert, insbesondere in führenden städtischen Krankenhäusern und Forschungszentren.
- Diese regionale Führungsrolle wird durch nationale Gesundheitsstrategien weiter gestärkt, die das Bewusstsein für seltene Krankheiten fördern, Patientenregister erweitern und die Zusammenarbeit zwischen lokalen Institutionen und internationalen Forschungseinrichtungen verbessern. Dadurch wird Saudi-Arabien zu einem zentralen Zentrum für die Diagnose und Behandlung von MHE in der gesamten MEA-Region.
Markteinblicke für multiple hereditäre Exostosen in Saudi-Arabien
Der saudi-arabische Markt für multiple hereditäre Exostosen erzielte 2024 den größten Umsatzanteil in der Region, angetrieben durch starke staatliche Investitionen in Genommedizin, spezialisierte orthopädische Versorgung und die Erforschung seltener Krankheiten. Die Einführung nationaler genetischer Screening-Programme und der Zugang zu fortschrittlicher Diagnostik wie MRT und CT haben die frühzeitige Erkennung und Behandlung von MHE deutlich verbessert. Darüber hinaus verbessern Partnerschaften zwischen saudischen Gesundheitseinrichtungen und internationalen Forschungsorganisationen die klinischen Ergebnisse und fördern Innovationen im Management seltener Krankheiten.
Markteinblick in die Vereinigten Arabischen Emirate (VAE) für multiple hereditäre Exostosen
Der Markt für multiple hereditäre Exostosen in den VAE wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen. Dies ist auf Fortschritte im Medizintourismus, ein gestiegenes Bewusstsein für genetische Erkrankungen und hohe Gesundheitsausgaben pro Kopf zurückzuführen. Die Verfügbarkeit erstklassiger Spezialkliniken und der frühzeitige Zugang zu chirurgischer Versorgung machen die VAE zu einem Zentrum der MHE-Behandlung in der Golfregion. Staatliche Programme zur Präzisionsmedizin und eine verstärkte Aufklärung der Öffentlichkeit über seltene Krankheiten unterstützen den Aufwärtstrend des Marktes zusätzlich.
Markteinblick in Südafrika für multiple hereditäre Exostosen
Der südafrikanische Markt für multiple hereditäre Exostose wächst stetig, da die Diagnostik seltener Krankheiten zunehmend im Fokus steht und der Zugang zu öffentlichen Gesundheitsdiensten verbessert wird. Die Integration von genetischer Beratung und bildgebender Diagnostik in Krankenhäuser der tertiären Versorgung hat die Erkennungsraten verbessert. Während ländliche Gebiete weiterhin Herausforderungen bieten, steigt in städtischen Zentren der Bedarf an kinderorthopädischer Versorgung, die für die Behandlung von MHE unerlässlich ist. Die Zusammenarbeit mit internationalen Organisationen trägt zudem dazu bei, die Forschungskapazitäten für seltene Krankheiten in Südafrika zu verbessern.
Markteinblick in Ägypten für multiple hereditäre Exostosen
Der ägyptische Markt für multiple hereditäre Exostosen gewinnt an Bedeutung, was auf die strategische Ausrichtung des Landes auf Gesundheitsreformen und die Berücksichtigung seltener Krankheiten in den nationalen Politikrahmen zurückzuführen ist. Universitätskliniken werden zunehmend mit Diagnosetechnologien wie Gentests und MRTs ausgestattet, die eine bessere Patientenidentifizierung ermöglichen. Öffentliche Gesundheitsinitiativen und Schulungsprogramme für Ärzte schärfen das Bewusstsein für die Krankheit, während Partnerschaften mit NGOs und regionalen Forschungseinrichtungen dazu beitragen, Versorgungslücken in unterversorgten Bevölkerungsgruppen zu schließen.
Marktanteile im Nahen Osten und Afrika bei multipler hereditärer Exostose
Die Branche der Behandlung multipler hereditärer Exostosen im Nahen Osten und Afrika wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Illumina, Inc. (USA)
- Medtronic (Irland)
- Zimmer Biomet Holdings, Inc. (USA)
- Stryker (USA)
- Siemens Healthineers AG (Deutschland)
- GE HealthCare Technologies Inc. (USA)
- Canon Medical Systems Corporation (Japan)
- Esaote SpA (Italien)
- Thermo Fisher Scientific Inc. (USA)
- Roche Holding AG (Schweiz)
- Koninklijke Philips NV (Niederlande)
- B. Braun Melsungen AG (Deutschland)
- Agilent Technologies, Inc. (USA)
- PerkinElmer Inc. (USA)
- FUJIFILM Corporation (Japan)
- Beijing Genomics Institute (BGI Genomics) (China)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- Jiangsu Trautec Medical Technology Co., Ltd. (China)
- Toshiba Corporation (Japan)
- Takara Bio Inc. (Japan)
Was sind die jüngsten Entwicklungen auf dem Markt für multiple hereditäre Exostose im Nahen Osten und in Afrika?
- Im Mai 2024 eröffnete das King Faisal Specialist Hospital and Research Centre (Saudi-Arabien) eine spezielle Klinik für seltene Knochenerkrankungen, die sich auf Erkrankungen wie multiple hereditäre Exostose konzentriert. Die Initiative zielt darauf ab, eine integrierte Versorgung durch genetische Beratung, orthopädische Interventionen und langfristige Nachsorge anzubieten. Dies spiegelt den wachsenden Fokus Saudi-Arabiens auf die Spezialisierung auf seltene Krankheiten und die Früherkennung bei Kindern wider.
- Im April 2024 ging Mediclinic Middle East (VAE) eine Kooperation mit einem internationalen Genomiklabor ein, um den Zugang zu EXT1- und EXT2-Genmutationstests zu verbessern und so eine frühzeitige und präzise Diagnose von MHE zu ermöglichen. Diese Partnerschaft stärkt die Kapazität lokaler Kliniken für Präzisionsmedizin und unterstreicht den Vorstoß der Region hin zu personalisierter Medizin.
- Im März 2024 startete das Groote Schuur Hospital (Südafrika) ein Pilotprogramm zur Integration der MRT-basierten Knorpelkappenüberwachung bei pädiatrischen MHE-Patienten. Diese Initiative soll die Operationsplanung und die langfristigen orthopädischen Ergebnisse verbessern und einen Wandel hin zu einer proaktiveren und datengesteuerten MHE-Versorgung im öffentlichen Gesundheitswesen einleiten.
- Im Februar 2024 startete das ägyptische Gesundheitsministerium eine landesweite Aufklärungskampagne zu genetisch bedingten Skeletterkrankungen, einschließlich MHE, in öffentlichen Krankenhäusern und Kinderkliniken. Die Kampagne konzentriert sich auf die frühzeitige Erkennung von Symptomen und Überweisungsprotokolle, um Diagnoseverzögerungen zu reduzieren und die Behandlungsergebnisse in unterversorgten Regionen zu verbessern.
- Im Januar 2024 startete CureRare Africa, eine gemeinnützige Allianz zur Unterstützung des Managements seltener Krankheiten, in Kenia und Nigeria ein Pilotprojekt für ein MHE-Register, um Prävalenz, Behandlungspfade und Patientenergebnisse zu erfassen. Dieser datenbasierte Ansatz unterstützt klinische Forschung und Lobbyarbeit, trägt dazu bei, Versorgungslücken auf dem Kontinent zu schließen und die Zusammenarbeit zwischen Gesundheitsdienstleistern, Forschern und politischen Entscheidungsträgern zu fördern.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 PIPELINE ANALYSIS - OBSERVATORY DATA
4.4 EPIDEMIOLOGY
5 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: REGULATIONS
5.1 REGULATION IN UNITED STATES: U.S. FOOD AND DRUG ADMINISTRATION (FDA)
5.1.1 REGULATION IN EUROPE: EUROPEAN MEDICINES AGENCY (EMA)
5.2 REGULATION IN ASIA-PACIFIC (JAPAN): PHARMACEUTICAL AND MEDICAL DEVICES AGENCY (PMDA)
5.3 MEDICAL DEVICE STANDARDS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF GENETIC DISORDERS
6.1.2 GROWING PEDIATRIC POPULATION
6.1.3 DEVELOPMENT OF NOVEL THERAPIES
6.2 RESTRAINTS
6.2.1 HIGH COST OF ADVANCED THERAPIES
6.2.2 LIMITED AVAILABILITY OF THERAPIES
6.3 OPPORTUNITIES
6.3.1 INCREASE IN PATIENT CARE AND SUPPORT SYSTEMS
6.3.2 INCREASE IN THE NUMBER OF COLLABORATIONS AND PARTNERSHIPS
6.4 CHALLENGES
6.4.1 LIMITED AWARENESS ABOUT THE DISORDER
6.4.2 LACK OF DRUG APPROVALS ASSOCIATED WITH THE DISORDER
7 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE
7.1 OVERVIEW
7.2 SESSILE
7.3 PEDUNCULATED
8 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT
8.1 OVERVIEW
8.2 SURGERY
8.2.1 REMOVE THE TUMOR
8.2.2 LENGTHEN LIMBS
8.3 MEDICATION
8.3.1 HOSPITAL PHARMACIES
8.3.2 DRUGS STORES AND RETAIL PHARMACIES
8.3.3 ONLINE PHARMACIES
8.4 OTHERS
9 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS
9.1 OVERVIEW
9.2 X-RAY
9.2.1 SESSILE
9.2.2 PEDUNCULATED
9.3 COMPUTED TOMOGRAPHY (CT) SCAN
9.3.1 SESSILE
9.3.2 PEDUNCULATED
9.4 MAGNETIC RESONANCE IMAGING (MRI)
9.4.1 SESSILE
9.4.2 PEDUNCULATED
9.5 GENETIC TESTS
9.5.1 SESSILE
9.5.2 PEDUNCULATED
9.6 OTHERS
9.6.1 SESSILE
9.6.2 PEDUNCULATED
10 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE
10.1 OVERVIEW
10.2 LEGS
10.3 ARMS
10.4 SHOULDERS
10.5 PELVIS
10.6 FINGERS
10.7 TOES
11 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
12 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.2.1 PRIVATE
12.2.2 GOVERNMENT
12.3 SPECIALTY CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 MIDDLE EAST AND AFRICA MULTIPLE HEREDITY EXOSTOSIS MARKET, BY REGION
13.1 MIDDLE EAST AND AFRICA
13.1.1 SAUDI ARABIA
13.1.2 U.A.E.
13.1.3 EGYPT
13.1.4 KUWAIT
13.1.5 QATAR
13.1.6 OMAN
13.1.7 BAHRAIN
13.1.8 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 BAYERS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT UPDATES
16.2 HALEON GROUP OF COMPANIES
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 BASF
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT UPDATES
16.4 VIATRIS INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT UPDATES
16.5 ACTIZAPHARMA
16.5.1 COMPANY SNAPSHOT
16.5.2 PRODUCT PORTFOLIO
16.5.3 RECENT UPDATES
16.6 ADVACARE PHARMA
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT UPDATES
16.7 AUROBINDO PHARMA
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT TABLETS
16.8 HALEON GROUP OF COMPANIES
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT UPDATES
16.9 IPSEN BIOPHARMACEUTICALS, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT UPDATES
16.1 MALLINCKRODT
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT UPDATES
16.11 TEVA PHARMACEUTICAL INDUSTRIES LTD.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT UPDATES
16.12 TAJ PHARMACEUTICALS LIMITED
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT UPDATES
17 QUESTIONNAIRE
18 RELATED REPORTS
Tabellenverzeichnis
TABLE 1 PIPELINE ANALYSIS - OBSERVATORY DATA
TABLE 2 PIPELINE ANALYSIS - INTERVENTIONAL DATA
TABLE 3 SALES DATA - 2024
TABLE 4 SALES DATA - 2023
TABLE 5 SALES DATA - 2022
TABLE 6 SALES DATA - 2021
TABLE 7 SALES DATA - 2020
TABLE 8 SALES DATA - 2019
TABLE 9 SALES DATA - 2018
TABLE 10 COST OF PALOVAROTENE
TABLE 11 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 12 MIDDLE EAST AND AFRICA SESSILE IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 13 MIDDLE EAST AND AFRICA PEDUNCULATED IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 14 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 15 MIDDLE EAST AND AFRICA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 16 MIDDLE EAST AND AFRICA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 17 MIDDLE EAST AND AFRICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 18 MIDDLE EAST AND AFRICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 19 MIDDLE EAST AND AFRICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 20 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 21 MIDDLE EAST AND AFRICA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 22 MIDDLE EAST AND AFRICA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 23 MIDDLE EAST AND AFRICA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 24 MIDDLE EAST AND AFRICA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 25 MIDDLE EAST AND AFRICA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 26 MIDDLE EAST AND AFRICA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 27 MIDDLE EAST AND AFRICA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 28 MIDDLE EAST AND AFRICA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 29 MIDDLE EAST AND AFRICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 30 MIDDLE EAST AND AFRICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 31 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 32 MIDDLE EAST AND AFRICA LEGS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 33 MIDDLE EAST AND AFRICA ARMS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 34 MIDDLE EAST AND AFRICA SHOULDERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 35 MIDDLE EAST AND AFRICA PELVIS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 36 MIDDLE EAST AND AFRICA FINGERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 37 MIDDLE EAST AND AFRICA TOES IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 38 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 39 MIDDLE EAST AND AFRICA PEDIATRIC IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 40 MIDDLE EAST AND AFRICA ADULT IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 41 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 42 MIDDLE EAST AND AFRICA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 43 MIDDLE EAST AND AFRICA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 44 MIDDLE EAST AND AFRICA SPECIALTY CLINICS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 45 MIDDLE EAST AND AFRICA AMBULATORY SURGICAL CENTERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 46 MIDDLE EAST AND AFRICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 47 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY COUNTRY, 2022-2031 (USD THOUSAND)
TABLE 48 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 49 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 50 MIDDLE EAST AND AFRICA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 51 MIDDLE EAST AND AFRICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 52 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 53 MIDDLE EAST AND AFRICA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 54 MIDDLE EAST AND AFRICA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 55 MIDDLE EAST AND AFRICA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 56 MIDDLE EAST AND AFRICA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 57 MIDDLE EAST AND AFRICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 58 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 59 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 60 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 61 MIDDLE EAST AND AFRICA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 62 SAUDI ARABIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 63 SAUDI ARABIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 64 SAUDI ARABIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 65 SAUDI ARABIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 66 SAUDI ARABIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 67 SAUDI ARABIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 68 SAUDI ARABIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 69 SAUDI ARABIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 70 SAUDI ARABIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 71 SAUDI ARABIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 72 SAUDI ARABIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 73 SAUDI ARABIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 74 SAUDI ARABIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 75 SAUDI ARABIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 76 SOUTH AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 77 SOUTH AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 78 SOUTH AFRICA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 79 SOUTH AFRICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 80 SOUTH AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 81 SOUTH AFRICA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 82 SOUTH AFRICA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 83 SOUTH AFRICA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 84 SOUTH AFRICA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 85 SOUTH AFRICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 86 SOUTH AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 87 SOUTH AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 88 SOUTH AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 89 SOUTH AFRICA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 90 U.A.E. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 91 U.A.E. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 92 U.A.E. SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 93 U.A.E. MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 94 U.A.E. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 95 U.A.E. X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 96 U.A.E. COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 97 U.A.E. MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 98 U.A.E. GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 99 U.A.E. OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 100 U.A.E. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 101 U.A.E. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 102 U.A.E. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 103 U.A.E. HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 104 EGYPT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 105 EGYPT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 106 EGYPT SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 107 EGYPT MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 108 EGYPT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 109 EGYPT X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 110 EGYPT COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 111 EGYPT MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 112 EGYPT GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 113 EGYPT OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 114 EGYPT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 115 EGYPT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 116 EGYPT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 117 EGYPT HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 118 KUWAIT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 119 KUWAIT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 120 KUWAIT SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 121 KUWAIT MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 122 KUWAIT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 123 KUWAIT X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 124 KUWAIT COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 125 KUWAIT MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 126 KUWAIT GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 127 KUWAIT OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 128 KUWAIT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 129 KUWAIT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 130 KUWAIT MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 131 KUWAIT HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 132 QATAR MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 133 QATAR MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 134 QATAR SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 135 QATAR MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 136 QATAR MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 137 QATAR X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 138 QATAR COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 139 QATAR MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 140 QATAR GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 141 QATAR OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 142 QATAR MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 143 QATAR MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 144 QATAR MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 145 QATAR HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 146 OMAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 147 OMAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 148 OMAN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 149 OMAN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 150 OMAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 151 OMAN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 152 OMAN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 153 OMAN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 154 OMAN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 155 OMAN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 156 OMAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 157 OMAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 158 OMAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 159 OMAN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 160 BAHRAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 161 BAHRAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 162 BAHRAIN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 163 BAHRAIN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 164 BAHRAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 165 BAHRAIN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 166 BAHRAIN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 167 BAHRAIN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 168 BAHRAIN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 169 BAHRAIN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 170 BAHRAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 171 BAHRAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 172 BAHRAIN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 173 BAHRAIN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 174 REST OF MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
Abbildungsverzeichnis
FIGURE 1 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: MIDDLE EAST AND AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 PRODUCT LIFELINE CURVE
FIGURE 9 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 10 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 12 EXECUTIVE SUMMARY
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 TWO SEGMENTS COMPRISE THE MIDDLE EAST AND AFRICA MULTIPLE HEREDITY EXOSTOSIS MARKET, BY TYPE
FIGURE 15 RISING PREVALENCE OF GENETIC DISORDERS IS DRIVING THE GROWTH OF THE MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET FROM 2024 TO 2031
FIGURE 16 THE TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET IN 2024 AND 2031
FIGURE 17 MARKET OVERVIEW
FIGURE 18 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2023
FIGURE 19 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2024-2031 (USD THOUSAND)
FIGURE 20 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, CAGR (2024-2031)
FIGURE 21 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2023
FIGURE 23 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2024-2031 (USD THOUSAND)
FIGURE 24 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, CAGR (2024-2031)
FIGURE 25 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 26 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2023
FIGURE 27 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2024-2031 (USD THOUSAND)
FIGURE 28 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, CAGR (2024-2031)
FIGURE 29 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, LIFELINE CURVE
FIGURE 30 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2023
FIGURE 31 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2024-2031 (USD THOUSAND)
FIGURE 32 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, CAGR (2024-2031)
FIGURE 33 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, LIFELINE CURVE
FIGURE 34 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2023
FIGURE 35 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2024-2031 (USD THOUSAND)
FIGURE 36 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, CAGR (2024-2031)
FIGURE 37 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 38 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2023
FIGURE 39 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2024-2031 (USD THOUSAND)
FIGURE 40 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, CAGR (2024-2031)
FIGURE 41 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 MIDDLE EAST AND AFRICA MULTIPLE HEREDITY EXOSTOSIS MARKET: SNAPSHOT (2023)
FIGURE 43 MIDDLE EAST AND AFRICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY SHARE 2023 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.