Global Viral Antigen Diagnostics Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
16.49 Billion
USD
30.30 Billion
2024
2032
USD
16.49 Billion
USD
30.30 Billion
2024
2032
| 2025 –2032 | |
| USD 16.49 Billion | |
| USD 30.30 Billion | |
|
|
|
|
Globale Marktsegmentierung für virale Antigendiagnostik nach Diagnosetest (Probenuntersuchung, serodiagnostische Tests und Virusisolierung), Virustyp (Adenovirus, Cytomegalovirus, Dengue-Virus, Enterovirus, Hepatitis-Virus, HIV-1, Coronavirus, Humanes Metapneumovirus, Humanes Rhinovirus A, Masernvirus, Poliovirus, Tollwutvirus, Varizella-Zoster-Virus, Vogelgrippe, Coxsackievirus, Epstein-Barr-Virus (EBV), Lymphokryptovirus, Herpesvirus (HSV-1, HSV-2), HIV-2, Humanes Herpesvirus, Humanes Papillomavirus (HPV), Influenzavirus, Mumpsvirus, Rötelnvirus, Respiratorisches Synzytialvirus (RSV) und West-Nil-Virus), Endbenutzer (Arztpraxen, kommerzielle Labore und Pflegeheime) – Branchentrends und Prognose bis 2032
Marktgröße für virale Antigendiagnostik
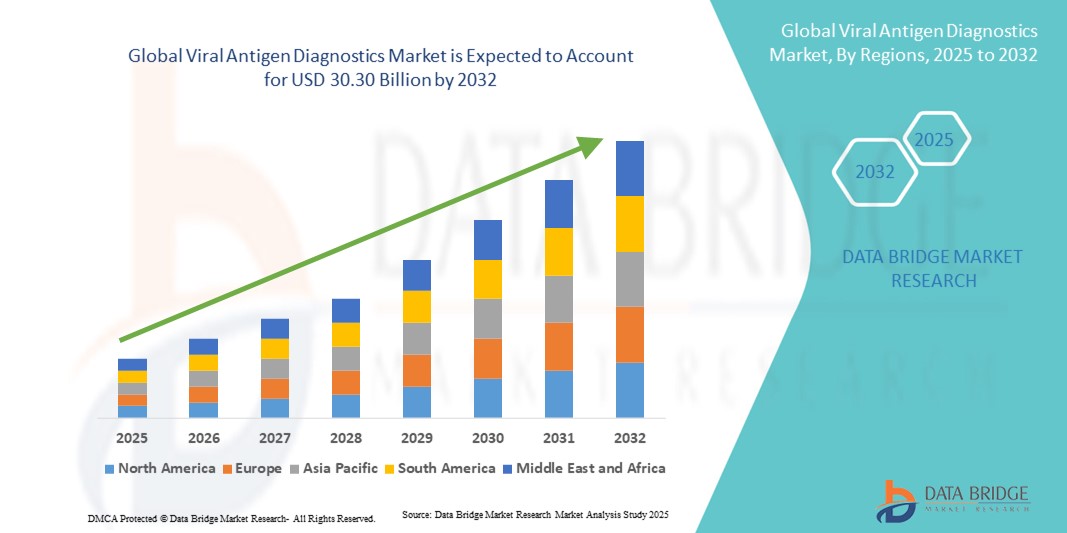
- Der globale Markt für virale Antigendiagnostik wird im Jahr 2024 auf 16,49 Milliarden US-Dollar geschätzt und soll bis 2032 30,30 Milliarden US-Dollar erreichen , bei einer CAGR von 7,90 % im Prognosezeitraum.
- Das Marktwachstum wird maßgeblich durch die weltweit zunehmende Verbreitung von Virusinfektionen und die wachsende Nachfrage nach schnellen, präzisen und patientennahen Diagnoselösungen in Krankenhäusern, Kliniken und Laboren vorangetrieben.
- Darüber hinaus treiben technologische Fortschritte in der Testentwicklung sowie ein wachsendes Bewusstsein für die Früherkennung und rechtzeitige Behandlung von Viruserkrankungen die Verbreitung viraler Antigendiagnostik voran. Diese konvergierenden Faktoren beschleunigen die Verbreitung schneller Diagnoselösungen und fördern damit das Wachstum der Branche erheblich.
Marktanalyse für virale Antigendiagnostik
- Die virale Antigendiagnostik ermöglicht einen schnellen und spezifischen Nachweis viraler Infektionen durch Antigenidentifizierung und ist aufgrund ihrer Schnelligkeit, Genauigkeit und Benutzerfreundlichkeit ein zunehmend wichtiger Bestandteil moderner Gesundheits- und Point-of-Care-Testsysteme in Krankenhäusern und Laboren.
- Die steigende Nachfrage nach viraler Antigendiagnostik wird vor allem durch die steigende Prävalenz viraler Infektionen, das wachsende Bewusstsein für die Vorteile einer Früherkennung und den starken Bedarf an zeitnahen, zuverlässigen Testlösungen im klinischen und kommunalen Umfeld angetrieben.
- Nordamerika dominierte den Markt für virale Antigendiagnostik mit dem größten Umsatzanteil von 39,2 % im Jahr 2024. Der Markt zeichnet sich durch eine fortschrittliche Gesundheitsinfrastruktur, hohe Gesundheitsausgaben und eine starke Präsenz führender Diagnostikunternehmen aus. In den USA kommt es zu einer starken Verbreitung von Antigentests, insbesondere in der Notfallversorgung und in kommunalen Testzentren, was auf Innovationen in der Testtechnologie und bei digitalen Berichtssystemen zurückzuführen ist.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum die am schnellsten wachsende Region im Markt für virale Antigendiagnostik sein. Grund hierfür sind steigende Investitionen im Gesundheitswesen, ein wachsendes Bewusstsein für Virusinfektionen und der Ausbau der diagnostischen Testinfrastruktur in Schwellenländern.
- Die Untersuchung von Proben dominierte den Markt für virale Antigendiagnostik mit einem Anteil von 42,8 % im Jahr 2024. Dies ist auf die Fähigkeit zurückzuführen, schnelle und genaue Ergebnisse direkt aus Patientenproben zu liefern, was sie ideal für Point-of-Care-Tests und zeitnahe klinische Entscheidungen macht.
Berichtsumfang und Marktsegmentierung für virale Antigendiagnostik
|
Eigenschaften |
Wichtige Markteinblicke zur viralen Antigendiagnostik |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für virale Antigendiagnostik
Fortschritte bei Schnelltests und Point-of-Care-Tests
- Ein bedeutender und sich beschleunigender Trend auf dem globalen Markt für virale Antigendiagnostik ist die Entwicklung schneller Point-of-Care-Testplattformen , die innerhalb weniger Minuten hochpräzise Ergebnisse liefern und so die Früherkennung und das Krankheitsmanagement verbessern.
- Beispielsweise ermöglicht die BinaxNOW COVID-19 Ag Card Gesundheitsdienstleistern, mit minimaler Ausrüstung und Schulung schnelle Antigentests in Kliniken und kommunalen Testzentren durchzuführen
- Die Integration digitaler Berichte und Smartphone-Konnektivität in Diagnosekits ermöglicht die Weitergabe von Ergebnissen in Echtzeit und die epidemiologische Nachverfolgung, wodurch das Patientenmanagement und die Überwachung von Krankheitsausbrüchen verbessert werden.
- Fortschrittliche Multiplex-Assays, die mehrere virale Antigene gleichzeitig erkennen können, optimieren diagnostische Arbeitsabläufe, verkürzen die Testzeit und steigern so die Effizienz im klinischen Umfeld.
- Dieser Trend zu schnellen, vernetzten und multiplexen viralen Antigentests verändert die Erwartungen an die Geschwindigkeit und Zuverlässigkeit der Diagnose. Unternehmen wie Abbott entwickeln tragbare Antigentestgeräte mit digitalen Berichtsfunktionen.
- Die Nachfrage nach schneller, benutzerfreundlicher und hochpräziser viraler Antigendiagnostik wächst in Krankenhäusern, Kliniken und öffentlichen Gesundheitsprogrammen rasant, da die Interessengruppen der Früherkennung und Ausbruchskontrolle Priorität einräumen.
Marktdynamik für virale Antigendiagnostik
Treiber
Zunehmende Prävalenz viraler Infektionen und Bewusstsein für Früherkennung
- Die weltweit steigende Prävalenz viraler Infektionen und das wachsende Bewusstsein für die Vorteile einer Früherkennung sind ein wesentlicher Treiber für die gestiegene Nachfrage nach viraler Antigendiagnostik.
- So wurde beispielsweise mit der Einführung des Sofia SARS Antigen FIA im Jahr 2024 die schnelle Point-of-Care-Erkennung in Krankenhäusern und ambulanten Einrichtungen hervorgehoben und die Akzeptanz gefördert.
- Da sich Gesundheitsdienstleister auf eine rechtzeitige Diagnose konzentrieren, um Komplikationen und Übertragungen zu reduzieren, bietet die virale Antigendiagnostik im Vergleich zu herkömmlichen molekularen Tests Schnelligkeit und Komfort
- Initiativen im Bereich der öffentlichen Gesundheit und Aufklärungskampagnen fördern routinemäßige Virustests, insbesondere in Hochrisikogruppen, und erhöhen so die Marktnachfrage
- Die Möglichkeit, Testlösungen in digitale Gesundheitsplattformen für Echtzeitüberwachung und -berichterstattung zu integrieren, stärkt die Akzeptanz im klinischen und kommunalen Gesundheitswesen weiter
- Steigende Investitionen in die Gesundheitsinfrastruktur und staatliche Programme zur Überwachung von Infektionskrankheiten dürften die weltweite Verbreitung viraler Antigendiagnostik weiter vorantreiben.
Einschränkung/Herausforderung
Bedenken hinsichtlich der Genauigkeit und Hürden bei der Einhaltung gesetzlicher Vorschriften
- Bedenken hinsichtlich falsch positiver oder falsch negativer Ergebnisse bei viralen Antigentests stellen eine erhebliche Herausforderung für eine breitere Marktakzeptanz dar, insbesondere bei wichtigen klinischen Entscheidungen
- So führten beispielsweise erste Berichte über eine geringere Sensitivität einiger Grippe-Antigentests dazu, dass Kliniker zögerten, sich ausschließlich auf antigenbasierte Ergebnisse zu verlassen.
- Um diese Bedenken auszuräumen, sind strenge behördliche Genehmigungen, eine kontinuierliche Qualitätsvalidierung und die Einhaltung internationaler Diagnosestandards erforderlich, um das Vertrauen der Gesundheitsdienstleister zu gewährleisten.
- Darüber hinaus können regional unterschiedliche regulatorische Rahmenbedingungen den Markteintritt neuer Diagnoseprodukte verlangsamen, insbesondere in Schwellenländern mit sich entwickelnden Gesundheitsrichtlinien.
- Kostenüberlegungen für hochempfindliche Antigentests können die Einführung in ressourcenbeschränkten Umgebungen ebenfalls behindern, obwohl Schnelltests im Allgemeinen günstiger sind als molekulare Alternativen
- Die Bewältigung dieser Herausforderungen durch verbesserte Testempfindlichkeit, strenge Validierung und die Anpassung an regulatorische Standards wird für ein nachhaltiges Wachstum im Markt für virale Antigendiagnostik von entscheidender Bedeutung sein
Marktumfang für virale Antigendiagnostik
Der Markt ist nach Diagnosetest, Virentyp und Endbenutzer segmentiert.
- Durch Diagnosetest
Der Markt für virale Antigendiagnostik ist auf Basis von Diagnosetests in Probenuntersuchungen, serodiagnostische Tests und Virusisolierung unterteilt. Das Segment Probenuntersuchungen dominierte den Markt mit dem größten Umsatzanteil von 42,8 % im Jahr 2024. Dies ist auf die Fähigkeit zurückzuführen, schnelle und genaue Ergebnisse direkt aus Patientenproben zu liefern, was es ideal für Point-of-Care-Tests macht. Krankenhäuser und Kliniken bevorzugen die Probenuntersuchung häufig aufgrund ihrer Schnelligkeit, Zuverlässigkeit und des geringen Gerätebedarfs. Das Segment profitiert zudem von der Integration in digitale Berichtssysteme, was das Patientenmanagement und die epidemiologische Nachverfolgung verbessert. Darüber hinaus sind die Methoden der Probenuntersuchung vielseitig, mit mehreren viralen Antigenen kompatibel und für diagnostische Hochdurchsatz-Workflows geeignet.
Das Segment der serodiagnostischen Tests wird voraussichtlich zwischen 2025 und 2032 mit 24,1 % das höchste Wachstum verzeichnen. Dies wird durch die zunehmende Nutzung im Frühscreening und in groß angelegten epidemiologischen Studien vorangetrieben. Serodiagnostische Tests sind äußerst nützlich, um Immunreaktionen auf Virusinfektionen zu erkennen und liefern zusätzliche Informationen für die Krankheitsüberwachung. Das wachsende Bewusstsein für ihre Rolle bei der Identifizierung früherer Expositionen und der Steuerung von Impfstrategien treibt das Marktwachstum weiter voran. Zunehmende Forschungsinitiativen und Fortschritte bei enzymgekoppelten und Lateral-Flow-Serodiagnostiktests tragen ebenfalls zum schnellen Wachstum dieses Segments bei.
- Nach Virentyp
Auf der Grundlage des Virustyps ist der Markt für virale Antigendiagnostik in Adenovirus, Cytomegalovirus, Dengue-Virus, Enterovirus, Hepatitis-Virus, HIV-1, Coronavirus, Humanes Metapneumovirus, Humanes Rhinovirus A, Masernvirus, Poliovirus, Tollwutvirus, Varizella-Zoster-Virus, Vogelgrippe, Coxsackievirus, Epstein-Barr-Virus (EBV), Lymphokryptovirus, Herpesvirus (HSV-1, HSV-2), HIV-2, Humanes Herpesvirus, Humanes Papillomavirus (HPV), Influenzavirus, Mumpsvirus, Rötelnvirus, Respiratorisches Synzytial-Virus (RSV) und West-Nil-Virus unterteilt . Das Segment Coronavirus dominierte den Markt mit einem Anteil von 29,8 % im Jahr 2024, getrieben durch den anhaltenden globalen Fokus auf COVID-19-Erkennungs- und Überwachungsprogramme. Die weite Verbreitung von Schnelltests auf Antigen für das Coronavirus in Krankenhäusern, Kliniken und kommunalen Testzentren trug erheblich zu diesem Anteil bei. Staatliche Screening-Programme, Point-of-Care-Testinitiativen und ein hohes öffentliches Bewusstsein haben die Marktdominanz des Virus gestärkt. Technologische Innovationen, die die Sensitivität und Spezifität von Coronavirus-Antigentests verbessern, stärken dieses Segment zusätzlich.
Das Dengue-Virus-Segment wird voraussichtlich von 2025 bis 2032 die höchste durchschnittliche jährliche Wachstumsrate verzeichnen, angetrieben durch die zunehmende Verbreitung in tropischen und subtropischen Regionen sowie verstärkte Initiativen im Bereich der öffentlichen Gesundheit. Der schnelle Nachweis von Dengue-Antigenen ermöglicht ein rechtzeitiges Eingreifen und die Eindämmung von Ausbrüchen. Investitionen in erschwingliche, tragbare Dengue-Diagnosekits und der Ausbau der Testinfrastruktur in Schwellenländern beschleunigen die Akzeptanz. Verbesserte Multiplex-Testplattformen, die Dengue und andere Virusinfektionen gleichzeitig erkennen, tragen ebenfalls zum hohen Wachstumspotenzial des Segments bei.
- Nach Endbenutzer
Der Markt für virale Antigendiagnostik ist nach Endnutzern in Arztpraxen, kommerzielle Labore und Pflegeheime unterteilt. Das Segment der kommerziellen Labore hatte im Jahr 2024 mit 45,5 % den größten Marktanteil. Dies ist auf die Fähigkeit der Labore zurückzuführen, große Testvolumina durchzuführen, auf den Zugang zu fortschrittlichen Diagnosetechnologien und auf Partnerschaften mit Gesundheitsdienstleistern für groß angelegte Virustestprogramme. Kommerzielle Labore profitieren zudem von Skaleneffekten, hoher Betriebseffizienz und der Möglichkeit, schnelle Durchlaufzeiten anzubieten, was sie zur bevorzugten Wahl für Krankenhäuser und Gesundheitsbehörden macht.
Das Segment der Arztpraxen wird voraussichtlich von 2025 bis 2032 mit 22,8 % das höchste Wachstum verzeichnen, was auf die zunehmende Nutzung von Point-of-Care-Tests im ambulanten Bereich zurückzuführen ist. Einfach anzuwendende Antigen-Testkits mit sofortiger Ergebnisaussage verbessern die Patientenversorgung und reduzieren den Bedarf an Laborbesuchen. Das wachsende Bewusstsein von Ärzten und Patienten für Früherkennung sowie die Weiterentwicklung kompakter und benutzerfreundlicher Testgeräte beschleunigen die Akzeptanz in diesem Segment weiter.
Regionale Analyse des Marktes für virale Antigendiagnostik
- Nordamerika dominierte den Markt für virale Antigendiagnostik mit dem größten Umsatzanteil von 39,2 % im Jahr 2024, gekennzeichnet durch eine fortschrittliche Gesundheitsinfrastruktur, hohe Gesundheitsausgaben und eine starke Präsenz führender Diagnostikunternehmen
- Gesundheitsdienstleister und öffentliche Gesundheitsbehörden in der Region legen Wert auf schnelle und genaue Tests und schätzen die Bequemlichkeit, Geschwindigkeit und Zuverlässigkeit der viralen Antigendiagnostik für eine zeitnahe Patientenversorgung und Ausbruchskontrolle.
- Diese breite Akzeptanz wird durch hohe Gesundheitsausgaben, robuste regulatorische Rahmenbedingungen und laufende Investitionen in Diagnosetechnologie weiter unterstützt, wodurch sich die virale Antigendiagnostik als bevorzugte Lösung in Krankenhäusern, Kliniken und kommunalen Testzentren etabliert hat.
Markteinblick in die virale Antigendiagnostik in den USA
Der US-Markt für virale Antigendiagnostik erzielte 2024 mit 82 % den größten Umsatzanteil in Nordamerika, angetrieben durch die weite Verbreitung von Schnell- und Point-of-Care-Tests. Gesundheitsdienstleister und öffentliche Gesundheitsprogramme legen zunehmend Wert auf die Früherkennung von Virusinfektionen, um die Übertragung zu reduzieren und die Behandlungsergebnisse zu verbessern. Die steigende Nachfrage nach tragbaren und einfach zu handhabenden Diagnosekits, kombiniert mit der Integration in digitale Berichts- und Krankenhausinformationssysteme, treibt den Markt zusätzlich an. Darüber hinaus tragen laufende Innovationen in der Testempfindlichkeit und bei Multiplex-Testplattformen erheblich zum Marktwachstum bei.
Einblicke in den europäischen Markt für virale Antigendiagnostik
Der europäische Markt für virale Antigendiagnostik wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen. Dies ist vor allem auf zunehmende staatliche Initiativen zur Infektionskontrolle und das zunehmende Bewusstsein für die Vorteile einer Frühdiagnose zurückzuführen. Die zunehmende Urbanisierung und die steigende Prävalenz viraler Infektionen fördern die Akzeptanz in Krankenhäusern, Kliniken und kommerziellen Laboren. Europäische Gesundheitsdienstleister sind zudem von der Benutzerfreundlichkeit, der schnellen Bearbeitung und der hohen Genauigkeit von Antigentests überzeugt. Die Region verzeichnet Wachstum in allen diagnostischen Anwendungsbereichen, darunter Routine-Screening, Ausbruchsmanagement und klinische Überwachung.
Markteinblicke für virale Antigendiagnostik in Großbritannien
Der britische Markt für virale Antigendiagnostik wird im Prognosezeitraum voraussichtlich mit einer bemerkenswerten jährlichen Wachstumsrate wachsen, getrieben durch die gestiegene Nachfrage nach effizienten und zeitnahen Virustests im klinischen und ambulanten Bereich. Zunehmende Bedenken hinsichtlich Ausbrüchen und Infektionskontrolle fördern die Akzeptanz in Krankenhäusern, Arztpraxen und Gesundheitsbehörden. Die fortschrittliche Gesundheitsinfrastruktur des Landes und der starke Fokus auf Früherkennung unterstützen das Marktwachstum. Die Integration in elektronische Patientenakten und Point-of-Care-Testinitiativen dürfte die Akzeptanz weiter fördern.
Markteinblicke für virale Antigendiagnostik in Deutschland
Der deutsche Markt für virale Antigendiagnostik wird im Prognosezeitraum voraussichtlich mit einer beträchtlichen jährlichen Wachstumsrate wachsen, angetrieben durch das Bewusstsein für Früherkennung und präventive Gesundheitsfürsorge. Das robuste deutsche Gesundheitssystem, hochwertige Diagnoselabore und die staatliche Unterstützung der Überwachung von Infektionskrankheiten fördern die Einführung viraler Antigentests. Die Integration der schnellen Antigendiagnostik in Krankenhausabläufe und Meldesysteme des öffentlichen Gesundheitswesens gewinnt zunehmend an Bedeutung. Die Nachfrage nach präzisen, hochempfindlichen Diagnoselösungen, die den lokalen regulatorischen Standards entsprechen, treibt das Marktwachstum weiter voran.
Markteinblicke für virale Antigendiagnostik im asiatisch-pazifischen Raum
Der Markt für virale Antigendiagnostik im asiatisch-pazifischen Raum wird im Prognosezeitraum von 2025 bis 2032 voraussichtlich mit einer durchschnittlichen jährlichen Wachstumsrate von 25 % wachsen. Dies ist auf steigende Investitionen im Gesundheitswesen, die zunehmende Verbreitung viraler Infektionen und den Ausbau der Testinfrastruktur in Ländern wie China, Japan und Indien zurückzuführen. Der zunehmende Fokus der Region auf Früherkennung und staatliche Initiativen zur Förderung von Point-of-Care-Tests treiben die Akzeptanz voran. Darüber hinaus erweitern die wachsenden Produktionskapazitäten im asiatisch-pazifischen Raum und die Verfügbarkeit erschwinglicher Antigen-Testkits den Zugang für eine breitere Bevölkerung.
Markteinblick in die virale Antigendiagnostik in Japan
Der japanische Markt für virale Antigendiagnostik gewinnt aufgrund der fortschrittlichen Gesundheitsinfrastruktur des Landes, des hohen Gesundheitsbewusstseins der Bevölkerung und der Bedeutung schneller Diagnoselösungen an Dynamik. Die zunehmende Zahl von Kliniken und Krankenhäusern, die Point-of-Care-Tests anbieten, sowie die Integration in elektronische Patientenakten und vernetzte Diagnoseplattformen tragen zur Akzeptanz bei. Japans alternde Bevölkerung erhöht die Nachfrage nach praktischen, präzisen und zeitnahen viralen Antigentests sowohl im stationären als auch im klinischen Umfeld zusätzlich.
Markteinblicke für virale Antigendiagnostik in Indien
Der indische Markt für virale Antigendiagnostik erzielte 2024 den größten Umsatzanteil im asiatisch-pazifischen Raum. Dies ist auf den verbesserten Zugang zur Gesundheitsversorgung, die zunehmende Verbreitung viraler Infektionen und den Ausbau der diagnostischen Testinfrastruktur zurückzuführen. Indien ist einer der größten Märkte für kostengünstige Point-of-Care-Antigentests und erfreut sich zunehmender Akzeptanz in Krankenhäusern, kommerziellen Laboren und kommunalen Gesundheitsprogrammen. Regierungsinitiativen zur Stärkung der Überwachung von Infektionskrankheiten und die Präsenz lokaler Hersteller, die kostengünstige Lösungen anbieten, sind wichtige Faktoren für den Markt in Indien.
Marktanteil der viralen Antigendiagnostik
Die Branche der viralen Antigendiagnostik wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Creative Diagnostics (USA)
- EUROIMMUN Medizinische Labordiagnostika AG (Deutschland)
- Alpha Diagnostic Intl. Inc. (USA)
- The Native Antigen Company (Großbritannien)
- BioFire Diagnostics (USA)
- Premier Medical (Indien)
- Innovative Diagnostics (Frankreich)
- Meridian Bioscience, Inc. (USA)
- VedaLab (Frankreich)
- DiaSorin SpA (Italien)
- Hotgen Biotech (China)
- Immudex (Dänemark)
- Revvity, Inc. (USA)
- Thermo Fisher Scientific Inc. (USA)
- F. Hoffmann-La Roche Ltd (Schweiz)
- Abbott (USA)
- Siemens Healthineers AG (Deutschland)
- BD (USA)
- QuidelOrtho Corporation (USA)
- GenMark Diagnostics (USA)
Was sind die jüngsten Entwicklungen auf dem globalen Markt für virale Antigendiagnostik?
- Im Juli 2025 führte Quest Diagnostics einen neuen diagnostischen Labortest für das Oropouche-Virus ein. Dieser nutzt die Polymerase-Kettenreaktion (PCR)-Technologie zum Nachweis viraler RNA in frühen Infektionsstadien. Im weiteren Verlauf des Quartals sind auch serologische Tests zum Antikörpernachweis geplant, um die diagnostischen Möglichkeiten für diese neu auftretende Infektionskrankheit zu erweitern.
- Im Juli 2025 entwickelten Forscher des Indian Institute of Science Education and Research (IISER) in Pune einen schnellen und kostengünstigen Diagnosetest, der sowohl COVID-19 als auch das Zika-Virus innerhalb von 20 Minuten nachweist, ohne dass ein spezielles Labor erforderlich ist. Der Test nutzt eine modifizierte synthetische RNA-Sequenz, einen sogenannten „Toehold Switch“, und erzeugt einen sichtbaren Farbwechsel auf einem kleinen Papierplättchen. Damit bietet er eine vielversprechende Lösung für Regionen mit eingeschränkter Gesundheitsinfrastruktur.
- Im Mai 2025 veröffentlichte SEKISUI Diagnostics den OSOM RSV-Test, einen schnellen immunchromatographischen Test zum qualitativen Nachweis des Nukleoprotein-Antigens des Respiratorischen Synzytialvirus (RSV) in Nasenabstrichproben. Dieser CLIA-freigestellte Test liefert Ergebnisse in nur 15 Minuten und eignet sich für Point-of-Care-Anwendungen. Er ermöglicht die rechtzeitige Diagnose und Behandlung von RSV-Infektionen.
- Im Juli 2023 stellte Roche Diagnostics India den Elecsys HCV Duo vor, den ersten vollautomatischen Immunoassay des Landes, der gleichzeitig Antigene und Antikörper des Hepatitis-C-Virus (HCV) aus einer einzigen Plasma- oder Serumprobe nachweisen kann. Dieser duale Nachweisansatz ermöglicht die frühzeitige Erkennung von Infektionen und die Überwachung chronischer Fälle und verbessert so die diagnostische Effizienz und das Patientenmanagement.
- Im Mai 2023 erteilte die US-amerikanische Food and Drug Administration (FDA) die Marktzulassung für das VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack und das Anti-SARS-CoV-2 Total Reagent Pack, beide hergestellt von Ortho-Clinical Diagnostics, Inc. Diese Tests sind für die Verwendung mit dem VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Calibrator konzipiert und erleichtern den Nachweis von SARS-CoV-2-Antikörpern und unterstützen die Beurteilung der Immunantwort bei Einzelpersonen.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.