Global Stroke Diagnostics Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
4.10 Billion
USD
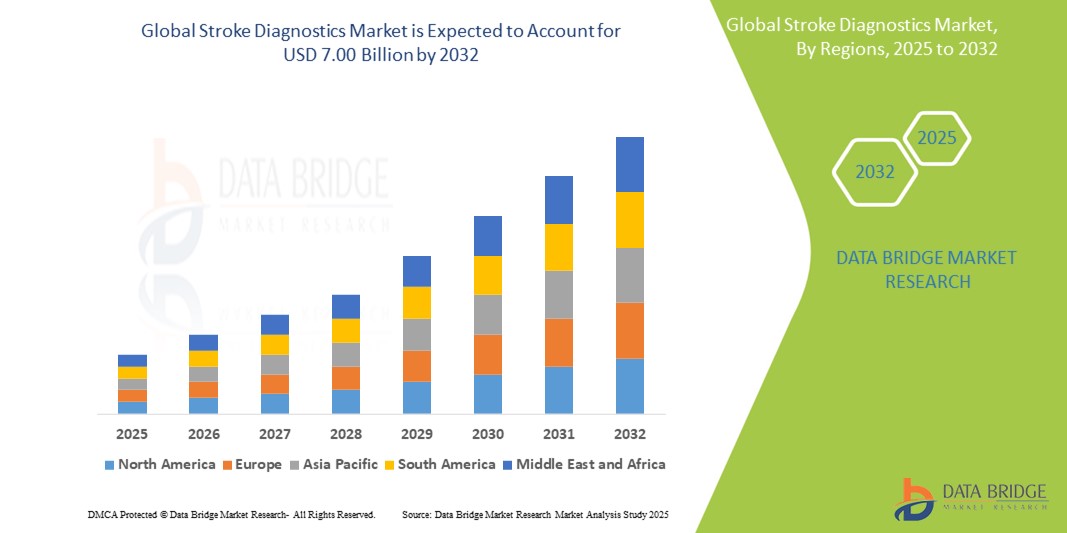
7.00 Billion
2024
2032
USD
4.10 Billion
USD
7.00 Billion
2024
2032
| 2025 –2032 | |
| USD 4.10 Billion | |
| USD 7.00 Billion | |
|
|
|
|
Globale Marktsegmentierung für Schlaganfalldiagnostik nach Schweregrad (mittelschwer, schwer und leicht), Typ (Diagnostik, Therapie und Software), Technologie (Computertomographie (CT-Scan), Computertomographie-Angiographie (CTA), Magnetresonanztomographie (MRT), Magnetresonanzangiographie (MRA), transkranieller Doppler-Ultraschall, Video-Kopfimpulstest (VHIT), Elektrokardiographie, Karotis-Ultraschall, zerebrale Angiographie und andere), Anwendung (ischämischer Schlaganfall, hämorrhagischer Schlaganfall und vorübergehende ischämische Attacken (TIAS)), Endbenutzer (Krankenhäuser, Kliniken, ambulante chirurgische Zentren und häusliche Gesundheitspflege), Vertriebskanal (Direktausschreibung, Drittanbieter und andere), Stadium (präoperativ, perioperativ und postoperativ) – Branchentrends und Prognose bis 2032
Wie groß ist der globale Markt für Schlaganfalldiagnostik und wie hoch ist seine Wachstumsrate?
- Der globale Markt für Schlaganfalldiagnostik wird im Jahr 2024 auf 4,10 Milliarden US-Dollar geschätzt und soll bis 2032 7,00 Milliarden US-Dollar erreichen , bei einer CAGR von 6,90 % im Prognosezeitraum.
- Das Marktwachstum wird vor allem durch die weltweit steigende Zahl von Schlaganfällen, das zunehmende Bewusstsein für Frühdiagnose und -behandlung sowie Fortschritte bei Diagnosetechnologien wie CT, MRT und Ultraschall vorangetrieben.
- Darüber hinaus beschleunigt die wachsende Nachfrage nach schnelleren, präziseren und nicht-invasiven Methoden zur Schlaganfallerkennung in Krankenhäusern, Kliniken und der häuslichen Pflege die Einführung innovativer Lösungen zur Schlaganfalldiagnose.
Was sind die wichtigsten Erkenntnisse des Marktes für Schlaganfalldiagnostik?
- Die Schlaganfalldiagnostik umfasst ein breites Spektrum an Technologien, darunter Bildgebungsverfahren (CT, MRT, CTA), Softwarelösungen und Diagnosegeräte, die auf eine frühzeitige und präzise Erkennung der Schwere und Art des Schlaganfalls abzielen.
- Steigende Gesundheitsausgaben, staatliche Initiativen zur Förderung des Schlaganfallbewusstseins und der Früherkennung sowie eine Verlagerung hin zur personalisierten Medizin sind Schlüsselfaktoren für das Marktwachstum
- Die Integration fortschrittlicher Bildgebungsverfahren mit KI-gestützter Software für verbesserte Genauigkeit und Arbeitsabläufe verändert die Schlaganfalldiagnostik weltweit.
- Nordamerika dominierte den Markt für Schlaganfalldiagnostik mit dem größten Umsatzanteil von 39,89 % im Jahr 2024, was auf eine weit verbreitete Gesundheitsinfrastruktur, fortschrittliche Diagnoseeinrichtungen und ein gestiegenes Bewusstsein für Schlaganfallmanagementtechnologien zurückzuführen ist.
- Der Markt für Schlaganfalldiagnostik im asiatisch-pazifischen Raum dürfte zwischen 2025 und 2032 mit 16,35 % die höchste jährliche Wachstumsrate verzeichnen. Dies ist auf das steigende Gesundheitsbewusstsein, die zunehmende Schlaganfallprävalenz und den Ausbau der Gesundheitsinfrastruktur in Ländern wie China, Indien und Japan zurückzuführen.
- Das Segment schwerer Schlaganfälle dominierte den Markt im Jahr 2024 und machte mit 47,6 % den größten Umsatzanteil aus. Grund dafür ist der dringende Bedarf an einer schnellen und genauen Diagnose, um rechtzeitig eingreifen zu können und die Sterblichkeit zu senken.
Berichtsumfang und Marktsegmentierung für Schlaganfalldiagnostik
|
Eigenschaften |
Wichtige Markteinblicke zur Schlaganfalldiagnostik |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Was ist der wichtigste Trend auf dem Markt für Schlaganfalldiagnostik?
„ KI-gestützte Bildgebung und prädiktive Diagnostik “
- Ein wichtiger Trend im Markt für Schlaganfalldiagnostik ist die Integration künstlicher Intelligenz (KI) in fortschrittliche bildgebende Verfahren zur Verbesserung der Diagnosegenauigkeit und -geschwindigkeit. KI-Algorithmen werden zunehmend eingesetzt, um ischämische oder hämorrhagische Schlaganfälle in CT- und MRT-Scans mit hoher Präzision und verkürzter Diagnosezeit zu erkennen.
- Beispielsweise lässt sich die Viz.ai-Plattform in Krankenhaussysteme integrieren und nutzt KI, um Schlaganfälle durch Verschluss großer Gefäße (LVO) in Echtzeit zu erkennen und Spezialisten sofort zu alarmieren, um die Behandlung zu priorisieren.
- Diese KI-basierten Diagnosetools beschleunigen die klinische Entscheidungsfindung und tragen dazu bei, menschliche Fehler zu reduzieren, insbesondere in Notfallsituationen, in denen Zeit entscheidend ist. Darüber hinaus werden prädiktive Modelle entwickelt, um das Schlaganfallrisiko anhand der Patientenanamnese und genetischer Marker zu bewerten.
- Auch der Trend zur Cloud-basierten KI-Diagnostik gewinnt an Dynamik und ermöglicht die Ferndiagnose von Schlaganfällen und die Unterstützung unterversorgter medizinischer Einrichtungen.
- Daher investieren Unternehmen wie RapidAI und Brainomix in die weltweite Erweiterung ihrer KI-basierten Schlaganfall-Bildgebungsplattformen, um die Zugänglichkeit und Genauigkeit zu verbessern.
- Diese Entwicklung hin zu einer prädiktiven, KI-gestützten Schlaganfalldiagnostik verändert den klinischen Ansatz in der Schlaganfallbehandlung und führt zu besseren Ergebnissen durch schnellere Interventionen und personalisiertere Behandlungsstrategien.
Was sind die Haupttreiber des Schlaganfalldiagnostikmarktes?
- Die weltweit steigende Zahl von Schlaganfällen, die auf die alternde Bevölkerung, einen sitzenden Lebensstil und die steigenden Raten von Bluthochdruck und Diabetes zurückzuführen ist, ist ein wichtiger Faktor, der die Nachfrage nach präziser Schlaganfalldiagnostik antreibt.
- So hat Brainomix beispielsweise im Februar 2024 seine KI-gestützte e-Stroke-Plattform auf mehrere Krankenhäuser in Großbritannien und Europa ausgeweitet, um die Früherkennung von Schlaganfällen und Behandlungsentscheidungen zu verbessern und so die Sterblichkeits- und Invaliditätsraten zu senken.
- Das wachsende Bewusstsein für die Vorteile einer frühzeitigen Schlaganfallerkennung, staatliche Gesundheitsreformen und erhöhte Investitionen in die neurologische Infrastruktur treiben den Markt weiter voran
- Der Vorstoß für nicht-invasive und schnelle Diagnoselösungen, gepaart mit Fortschritten in der Bildgebungstechnologie wie Perfusions-CT und diffusionsgewichteter MRT, unterstützt ebenfalls das Wachstum
- Darüber hinaus trägt die Integration von Telemedizin und mobilen Schlaganfallzentren dazu bei, die Schlaganfalldiagnostik in entlegene und unterversorgte Gebiete zu bringen, die Chancengleichheit im Gesundheitswesen zu verbessern und die Marktexpansion voranzutreiben.
Welcher Faktor stellt das Wachstum des Marktes für Schlaganfalldiagnostik in Frage?
- Eine große Herausforderung ist der eingeschränkte Zugang zu modernen Diagnosegeräten in Ländern mit niedrigem und mittlerem Einkommen, in denen es an Gesundheitsinfrastruktur und ausgebildetem Fachpersonal mangelt.
- So sind viele ländliche Krankenhäuser noch immer auf veraltete CT-Geräte angewiesen oder verfügen nicht über die nötigen Spezialisten zur Interpretation von Schlaganfallbildern, was zu verzögerten oder falschen Diagnosen führt.
- Darüber hinaus können die hohen Kosten für Schlaganfall-Diagnoseinstrumente, insbesondere KI-gestützte Plattformen und fortschrittliche Bildgebungssysteme, für kleinere Krankenhäuser oder Gesundheitssysteme mit eingeschränktem Budget unerschwinglich sein.
- Datenschutzbedenken im Zusammenhang mit KI-basierten Schlaganfall-Plattformen, insbesondere solchen, die Cloud-Speicher und Fernzugriff nutzen, haben sich in bestimmten Regionen ebenfalls als Hindernis für die Einführung herausgestellt.
- Die Bewältigung dieser Probleme durch kostengünstige Lösungen, skalierbare KI-Plattformen und verbesserte Schulungsinitiativen ist für die Ausweitung der Marktdurchdringung und die Verbesserung der Patientenergebnisse weltweit von entscheidender Bedeutung.
- Die Bewältigung dieser Herausforderungen durch verbesserte Cybersicherheitsmaßnahmen, Aufklärung der Verbraucher über bewährte Sicherheitspraktiken und die Entwicklung erschwinglicherer Optionen für die Schlaganfalldiagnostik wird für ein nachhaltiges Marktwachstum von entscheidender Bedeutung sein.
Wie ist der Markt für Schlaganfalldiagnostik segmentiert?
Der Markt ist nach Typ, Kommunikationsprotokoll, Entsperrmechanismus und Anwendung segmentiert.
- Nach Schweregrad
Der Markt für Schlaganfalldiagnostik wird nach Schweregrad in mittelschwere, schwere und leichte Schlaganfälle unterteilt. Das Segment der schweren Schlaganfälle dominierte den Markt im Jahr 2024 mit 47,6 % den größten Umsatzanteil. Grund dafür ist der dringende Bedarf an einer schnellen und präzisen Diagnose, um rechtzeitig eingreifen und die Sterblichkeit senken zu können. Schwere Schlaganfälle erfordern typischerweise fortschrittliche Diagnoseinstrumente für dringende Therapieentscheidungen, was die Nachfrage in diesem Segment ankurbelt.
Es wird erwartet, dass die Segmente mit mittlerem und leichtem Schweregrad stetig wachsen werden, da sich das Bewusstsein und die Bemühungen zur Früherkennung weltweit verbessern.
- Nach Typ
Der Markt ist nach Typ in Diagnostik, Therapie und Software segmentiert. Das Diagnostiksegment führte den Markt im Jahr 2024 mit einem beeindruckenden Umsatzanteil von 52,3 % an, was die zunehmende Nutzung fortschrittlicher bildgebender und KI-basierter Diagnoselösungen zur Schlaganfallfrüherkennung widerspiegelt.
Es wird erwartet, dass der Markt für Therapeutika und Softwarelösungen deutlich wachsen wird, insbesondere da integrierte Plattformen, die Diagnose mit Behandlungsplanung und -überwachung kombinieren, immer häufiger zum Einsatz kommen.
- Nach Technologie
Basierend auf der Technologie ist der Markt in Computertomographie (CT), Computertomographie-Angiographie (CTA), Magnetresonanztomographie (MRT), Magnetresonanzangiographie (MRA), Transkranieller Doppler-Ultraschall, Video-Kopfimpulstest (VHIT), Elektrokardiographie, Karotis-Ultraschall, Zerebrale Angiographie und weitere segmentiert. Das Segment Computertomographie (CT) hatte im Jahr 2024 mit 45,8 % den größten Marktanteil, was auf seine breite Verfügbarkeit, die schnellen Bildgebungsmöglichkeiten und seine entscheidende Rolle bei der Notfalldiagnostik von Schlaganfällen zurückzuführen ist.
Bei fortschrittlichen Bildgebungsverfahren wie CTA und MRT wird aufgrund ihrer höheren Sensibilität und zunehmenden klinischen Anwendung ein erhebliches Wachstum erwartet.
- Nach Anwendung
Der Markt ist nach Anwendung in ischämischer Schlaganfall, hämorrhagischer Schlaganfall und transitorische ischämische Attacken (TIAs) segmentiert. Das Segment des ischämischen Schlaganfalls dominierte im Jahr 2024 mit einem Marktanteil von 54,1 %, was auf seine weltweit höhere Prävalenz und den dringenden Bedarf an rechtzeitiger Diagnose und Behandlung zurückzuführen ist.
Auch in den Segmenten Hämorrhagischer Schlaganfall und TIA wird mit einem Wachstum gerechnet, da die diagnostische Präzision zunimmt und die Präventivbehandlung immer weiter verbreitet ist.
- Nach Endbenutzer
Der Markt ist nach Endverbrauchern in Krankenhäuser, Kliniken, ambulante Operationszentren und häusliche Pflege unterteilt. Das Krankenhaussegment erzielte im Jahr 2024 mit 60,5 % den größten Umsatzanteil, was auf die Konzentration fortschrittlicher Diagnoseeinrichtungen und Schlaganfallstationen in Krankenhäusern zurückzuführen ist.
Kliniken und ambulante Zentren erweitern ihre Möglichkeiten zur Schlaganfalldiagnose, während die häusliche Gesundheitsversorgung mit tragbaren und Fernüberwachungslösungen auf dem Vormarsch ist
- Nach Vertriebskanal
Basierend auf den Vertriebskanälen ist der Markt in Direktausschreibungen, Drittanbieter und andere segmentiert. Das Direktausschreibungssegment führte den Markt im Jahr 2024 mit einem Anteil von 48,7 % an, angetrieben von institutionellen Beschaffungspraktiken in Krankenhäusern und großen Gesundheitsorganisationen, die direkte Lieferantenbeziehungen für bessere Preise und besseren Service priorisieren. Drittanbieter gewinnen insbesondere in Schwellenländern an Boden.
- Nach Phase
Der Markt ist je nach Stadium in die präoperative, perioperative und postoperative Phase unterteilt. Das präoperative Segment dominierte im Jahr 2024 mit einem Umsatzanteil von 50,3 %, da eine frühzeitige Schlaganfalldiagnose und Risikobewertung für die Festlegung von Behandlungspfaden und die Verbesserung der Patientenergebnisse von entscheidender Bedeutung sind.
Auch die perioperativen und postoperativen Segmente wachsen mit Fortschritten in der Überwachungs- und Rehabilitationstechnologie.
Welche Region hält den größten Anteil am Markt für Schlaganfalldiagnostik?
- Nordamerika dominierte den Markt für Schlaganfalldiagnostik mit dem größten Umsatzanteil von 39,89 % im Jahr 2024, was auf eine weit verbreitete Gesundheitsinfrastruktur, fortschrittliche Diagnoseeinrichtungen und ein gestiegenes Bewusstsein für Schlaganfallmanagementtechnologien zurückzuführen ist.
- Die Region profitiert von hohen Gesundheitsausgaben, staatlichen Initiativen zur Förderung der Schlaganfallfrüherkennung und der zunehmenden Nutzung KI-gestützter Diagnoseinstrumente und Telemedizindienste.
- Diese Faktoren steigern gemeinsam die Nachfrage nach Schlaganfalldiagnostik in Krankenhäusern, Kliniken und im häuslichen Pflegebereich sowohl in den USA als auch in Kanada.
Markteinblick in die Schlaganfalldiagnostik in den USA
Der US-Markt für Schlaganfalldiagnostik erzielte 2024 den größten Umsatzanteil innerhalb Nordamerikas, angetrieben durch umfangreiche Investitionen in Forschung und Entwicklung und die schnelle Einführung fortschrittlicher Bildgebungsverfahren wie CT und MRT. Die zunehmende Zahl von Schlaganfällen und die alternde Bevölkerung erhöhen zudem die Nachfrage nach frühzeitiger und präziser Diagnose. Der Fokus des US-Gesundheitssystems auf die Reduzierung der Schlaganfall-bedingten Morbidität durch rechtzeitige Diagnostik unterstützt das Marktwachstum, ebenso wie die zunehmende Integration von Telemedizin und KI-gestützter Diagnosesoftware.
Markteinblick in die Schlaganfalldiagnostik in Europa
Der europäische Markt für Schlaganfalldiagnostik wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen, unterstützt durch die zunehmende staatliche Förderung von Schlaganfall-Aufklärungsprogrammen und die Finanzierung des Gesundheitswesens. Länder wie Deutschland, Frankreich und Großbritannien verzeichnen dank technologischer Fortschritte bei Bildgebungs- und Diagnoseverfahren ein Wachstum. Die zunehmende Urbanisierung und die alternde Bevölkerung treiben die Nachfrage weiter an, während strenge Gesundheitsvorschriften die Einführung hochwertiger Schlaganfalldiagnostiklösungen sicherstellen.
Markteinblick in die Schlaganfalldiagnostik in Großbritannien
Der britische Markt für Schlaganfalldiagnostik wird voraussichtlich mit einer bemerkenswerten jährlichen Wachstumsrate wachsen, angetrieben durch die Initiativen des National Health Service (NHS) zur Verbesserung der Schlaganfallbehandlungspfade und zur Förderung frühzeitiger Interventionen. Die steigende Zahl von Schlaganfällen und der zunehmende Einsatz KI-basierter Diagnostik zur Verkürzung der Behandlungszeit sind wichtige Wachstumsfaktoren. Darüber hinaus unterstützen eine verstärkte Finanzierung der Schlaganfallversorgung in der Gemeinde und ein wachsendes Bewusstsein für Schlaganfallsymptome das Marktwachstum.
Markteinblick in die Schlaganfalldiagnostik in Deutschland
Der deutsche Markt für Schlaganfalldiagnostik wird voraussichtlich deutlich wachsen, unterstützt durch die fortschrittliche Gesundheitsinfrastruktur und den Fokus auf Innovationen in der Medizintechnik. Das Engagement Deutschlands für die digitale Gesundheitstransformation und die steigende Schlaganfallrate tragen zu einer steigenden Nachfrage nach diagnostischen Bildgebungs- und Softwarelösungen bei. Öffentliche und private Investitionen im Gesundheitswesen fördern die Einführung modernster Schlaganfalldiagnostik in Krankenhäusern und der ambulanten Versorgung zusätzlich.
Welche Region ist die am schnellsten wachsende Region im Markt für Schlaganfalldiagnostik?
Der Markt für Schlaganfalldiagnostik im asiatisch-pazifischen Raum dürfte zwischen 2025 und 2032 mit 16,35 % die höchste durchschnittliche jährliche Wachstumsrate (CAGR) verzeichnen. Dies ist auf das steigende Gesundheitsbewusstsein, die zunehmende Schlaganfallprävalenz und den Ausbau der Gesundheitsinfrastruktur in Ländern wie China, Indien und Japan zurückzuführen. Regierungsinitiativen zur Förderung digitaler Gesundheitsversorgung und Telemedizin sowie die rasante Urbanisierung und die steigenden Gesundheitsausgaben der Mittelschicht beschleunigen das Marktwachstum. Darüber hinaus entwickelt sich die Region zu einem wichtigen Zentrum für Produktion und Innovation im Bereich der Diagnosetechnologien, wodurch die Zugänglichkeit und Erschwinglichkeit verbessert werden.
Markteinblick in die Schlaganfalldiagnostik in Japan
Der japanische Markt für Schlaganfalldiagnostik gewinnt aufgrund der alternden Bevölkerung und des fortschrittlichen Gesundheitssystems an Bedeutung. Die hohe Akzeptanz innovativer Medizinprodukte und KI-gestützter Diagnosetools im Land unterstützt das Marktwachstum. Die Integration der Schlaganfalldiagnostik mit Telemedizindiensten und Patientenfernüberwachung trägt dem wachsenden Bedarf an effizienter Schlaganfallversorgung, insbesondere in ländlichen Gebieten, Rechnung. Japans Fokus auf präventive Gesundheitsfürsorge und das Management chronischer Krankheiten treibt das Wachstum dieses Marktes weiter voran.
Markteinblick in die Schlaganfalldiagnostik in China
China hatte 2024 den größten Marktanteil im asiatisch-pazifischen Raum, was auf die rasante Urbanisierung, eine wachsende ältere Bevölkerung und steigende Gesundheitsausgaben zurückzuführen ist. Der starke Fokus der Regierung auf Schlaganfallpräventionsprogramme und Smart-Hospital-Initiativen unterstützt die zunehmende Verbreitung fortschrittlicher Diagnoselösungen. Darüber hinaus machen inländische Produktionskapazitäten und die wachsende Gesundheitsinfrastruktur die Schlaganfalldiagnostik in städtischen und ländlichen Regionen zugänglicher und treiben das Marktwachstum voran.
Welches sind die Top-Unternehmen auf dem Markt für Schlaganfalldiagnostik?
Die Schlaganfalldiagnostikbranche wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Siemens (Deutschland)
- Koninklijke Philips NV (Niederlande)
- GE HealthCare (USA)
- Mindray Medical India Pvt. Ltd. (Indien)
- FUJIFILM Corporation (Japan)
- CANON MEDICAL SYSTEMS CORPORATION (Japan)
- ALPINION MEDICAL SYSTEMS Co., Ltd. (Südkorea)
- Analogic Corporation (USA)
- Aspect Imaging Ltd. (USA)
- BPL Medical Technologies (Indien)
- Carestream Health (USA)
- Esaote SpA (Italien)
- FONAR Corp. (USA)
- Hologic, Inc. (USA)
- IMRIS Inc. (Kanada)
- Medfield Diagnostics AB (Schweden)
- MEDTRON AG (Deutschland)
Was sind die jüngsten Entwicklungen auf dem globalen Markt für Schlaganfalldiagnostik?
- Im Mai 2021 brachte Siemens den Somatom X.ceed auf den Markt, einen hochauflösenden Hochgeschwindigkeits-CT-Scanner für anspruchsvolle klinische Bereiche. Diese Innovation erweitert die Produktpalette von Siemens und unterstreicht das Engagement des Unternehmens für die Weiterentwicklung diagnostischer Bildgebungstechnologien. Der Somatom X.ceed ist auf präzise und effiziente Bildgebung zugeschnitten und erfüllt die komplexen Anforderungen verschiedener klinischer Szenarien.
- Im April 2021 erteilte die FDA die Zulassung für das Neurolutions IpsiHand Rehabilitationssystem für die oberen Extremitäten von Neurolutions, Inc. Dieses Brain-Computer-Interface (BCI)-Gerät erleichtert die Rehabilitation von Schlaganfallpatienten mit Behinderungen der oberen Extremitäten, insbesondere von Hand-, Handgelenk- und Armbeeinträchtigungen. Die Zulassung unterstreicht sein Potenzial zur Verbesserung der neurorehabilitativen Ergebnisse bei Patienten, die sich von schlaganfallbedingten motorischen Defiziten erholen.
- Im April 2021 gab Koninklijke Philips NV eine strategische Partnerschaft mit Ibex Medical Analytics bekannt, um gemeinsam die digitalen Pathologie- und KI-Lösungen weltweit voranzutreiben. Ziel dieser Zusammenarbeit ist es, die Vertriebskanäle zu stärken und Krankenhäusern, Gesundheitsnetzwerken und Pathologielaboren weltweit einen besseren Zugang zu innovativen Pathologielösungen zu ermöglichen.
- Im September 2020 erhielt Hyperfine Research, Inc. die FDA 510(k)-Zulassung für sein bahnbrechendes tragbares MR-Bildgebungssystem Swoop. Diese tragbare MRT-Technologie definiert die Point-of-Care-Bildgebung neu und ermöglicht eine sofortige Magnetresonanztomographie. Die FDA-Zulassung stellt einen bedeutenden Fortschritt dar und ermöglicht eine zugänglichere und schnellere diagnostische Bildgebung in verschiedenen Gesundheitseinrichtungen.
- Im Juni 2020 erhielt RapidAI die FDA-Zulassung für KI-Algorithmen, die CT-Scans des Gehirns schnell analysieren und mutmaßliche Verschlüsse großer Gefäße – eine der Hauptursachen für schwere Schlaganfälle – identifizieren können. Darüber hinaus erhielt das Unternehmen die FDA-Zulassung für computergestützte Diagnosesoftware, die Ärzten die Interpretation von CT-Scans ohne Kontrastmittel verbessert und so den Fortschritt in der Schlaganfalldiagnostik widerspiegelt.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.