Global Prosthetic Heart Valve Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1.12 Billion
USD
1.95 Billion
2024
2032
USD
1.12 Billion
USD
1.95 Billion
2024
2032
| 2025 –2032 | |
| USD 1.12 Billion | |
| USD 1.95 Billion | |
|
|
|
|
Global Prosthetic Heart Valve Market Segmentation, By Type (Transcatheter Heart Valve, Tissue Heart Valve, and Mechanical Heart Valve), Product (Repair Products, Mitral Valve Repair Devices, and Tricuspid Valve Repair Devices), Technology (Biological Valve and Decellularized Valve) End Use (Hospitals, Ambulatory Surgical Centres, Speciality Clinics, and Others) - Industry Trends and Forecast to 2032
Prosthetic Heart Valve Market Size
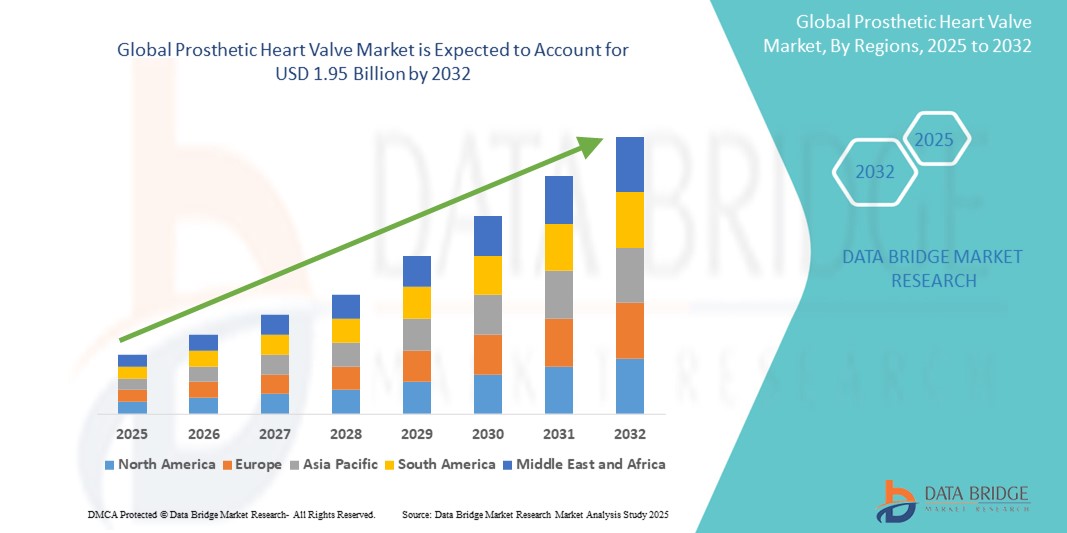
- The global prosthetic heart valve market size was valued atUSD 1.12 billion in 2024and is expected to reachUSD 1.95 billion by 2032, at aCAGR of 6.14%during the forecast period
- This growth is driven by factors such as the rising prevalence of valvular heart diseases, increasing geriatric population, advancements in prosthetic valve technologies, and growing demand for minimally invasive surgical procedures
Prosthetic Heart Valve Market Analysis
- The global prosthetic heart valve market is growing due to increased use of minimally invasive surgical methods that ensure quicker recovery
- These procedures are gaining traction among surgeons for offering reduced hospital stays and fewer post-surgery complications
- North America is expected to dominate the prosthetic heart valves market with 41.05% share due to advanced healthcare infrastructure and high prevalence of cardiovascular diseases
- Asia-Pacific is expected to be the fastest growing region in the prosthetic heart valve market with 12.4% market share during the forecast period due to rapid modernization of healthcare infrastructure and increasing per capita incomes
- The transcatheter heart valve segment is expected to dominate the prosthetic heart valve market with the largest share of 62.05% in 2025 due to its minimally invasive nature, which offers quicker recovery times and reduced risk compared to traditional open-heart surgeries
Report Scope and Prosthetic Heart Valve Market Segmentation
|
Attributes |
Prosthetic Heart Valve Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Prosthetic Heart Valve Market Trends
“Rise of Minimally Invasive Heart Valve Solutions”
- Transcatheter heart valves are becoming a key trend in the prosthetic heart valve market, offering a less invasive alternative to open-heart surgery
- These valves are increasingly preferred by patients and doctors due to faster recovery times and fewer post-operative complications
- Ongoing improvements in transcatheter valve technology are enhancing precision and reliability during implantation procedures
- Healthcare providers are expanding their use of these valves in a wider range of patients, including those once considered inoperable
- For instance, the launch of self-expanding and repositionable valve systems has allowed for more flexible and patient-specific treatments
- In conclusion, this growing trend highlights the shift toward innovative, patient-friendly cardiac care solutions in the current market
Prosthetic Heart Valve Market Dynamics
Driver
“Increasing Geriatric Population with Valve Disorders”
- The rising global geriatric population is increasing cases of valvular disorders such as aortic stenosis and mitral regurgitation, creating higher demand for prosthetic heart valves
- Older adults often have comorbidities that make traditional surgery risky, leading to a preference for advanced prosthetic valves with better safety and compatibility
- Healthcare systems are expanding cardiology departments and investing in infrastructure to manage the growing volume of elderly heart patients, especially in countries such as Japan and Germany
- For instance, the U.S. Centers for Disease Control and Prevention reports that heart valve disease affects one in eight people over age 75, reinforcing the need for valve solutions
- Manufacturers are developing valves suited to the anatomy and health profiles of older individuals, helping drive long-term market innovation and adoption
- In conclusion, as elderly populations continue to grow worldwide, the market for prosthetic heart valves is set to expand consistently
Opportunity
“Rising Adoption in Emerging Economies”
- Emerging economies in Asia, Latin America, and Africa are improving healthcare infrastructure, offering a growing opportunity for prosthetic heart valve adoption
- Governments and private healthcare providers are investing in building hospitals and equipping them with advanced tools for heart valve replacement surgeries
- For instance, India is seeing a rise in medical tourism as patients from neighboring regions seek affordable, high-quality heart valve procedures
- Local governments are introducing public health initiatives and insurance schemes to reduce the out-of-pocket costs for valve replacements, improving accessibility for a larger population
- Medical device companies are adapting their product offerings and pricing strategies to cater to the unique needs and demands of emerging economies
- In conclusion, as healthcare systems and economic conditions improve, emerging markets present a significant opportunity for growth in the prosthetic heart valve market
Restraint/Challenge
“High Cost and Limited Affordability”
- The high cost of prosthetic heart valves and related surgical procedures remains a significant barrier, limiting access to treatment for many patients
- Valve replacements require not only the device itself but also skilled surgeons, advanced facilities, and intensive post-operative care, all of which drive up costs
- For instance, bioprosthetic valves are made from expensive biological materials and require extensive testing, raising their overall price
- In regions with limited insurance coverage or inadequate public healthcare funding, patients often delay or avoid treatment due to financial constraints
- Many healthcare systems prioritize other urgent issues, resulting in insufficient funds allocated to heart valve treatments, further restricting access for low-income populations
- In conclusion, until the cost of valves and procedures decreases or affordable financing options are introduced, high prices will continue to hinder market growth worldwide
Prosthetic Heart Valve Market Scope
The market is segmented on the basis of type, product, technology, and end use.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Product |
|
|
By Technology |
|
|
By End Use
|
|
In 2025, the transcatheter heart valve segment is projected to dominate the market with a largest share in type segment
The transcatheter heart valve segment is expected to dominate the prosthetic heart valve market with the largest share of 62.05% in 2025 due to its minimally invasive nature, which offers quicker recovery times and reduced risk compared to traditional open-heart surgeries. This procedure allows for valve implantation through a catheter, eliminating the need for chest incisions and reducing hospital stays. Additionally, advancements in valve design, such as self-expanding and balloon-expandable valves, have further improved the safety and efficacy of transcatheter heart valve procedures.
The mitral valve repair devices segment is expected to account for the largest share during the forecast period in product segment
In 2025, the mitral valve repair devices segment is expected to dominate the market with the largest market share of 38.05% due to the increasing prevalence of mitral valve diseases, such as mitral regurgitation, which often require repair rather than replacement. The growing adoption of minimally invasive procedures for mitral valve repair, which offer reduced recovery times and lower complication risks, is driving the segment's growth.
Prosthetic Heart Valve Market Regional Analysis
“North America Holds the Largest Share in the Prosthetic Heart Valve Market”
- North America holds the largest share of the global prosthetic heart valve market, with 41.05% share due to advanced healthcare infrastructure and high prevalence of cardiovascular diseases
- The U.S., in particular, contributes significantly to this dominance, with major medical device companies headquartered there, such as Edwards Lifesciences, Medtronic, and Abbott
- Favorable reimbursement policies and strong regulatory frameworks support the adoption of advanced valve technologies, including transcatheter aortic valve replacement (TAVR) procedures
- The region's well-established research and development capabilities foster continuous innovation in prosthetic heart valve designs and materials
- Despite high healthcare costs, the demand for high-quality cardiac care sustains market growth, with a growing aging population further increasing the need for valve replacements
“Asia-Pacific is Projected to Register the Highest CAGR in the Prosthetic Heart Valve Market”
- The Asia-Pacific region is experiencing the highest growth rate with 12.4% market share in the prosthetic heart valve market, due to rapid modernization of healthcare infrastructure and increasing per capita incomes
- Countries such as China, India, and Japan are witnessing a rise in the incidence of valvular heart diseases, leading to higher demand for prosthetic heart valves
- Government initiatives, such as national health insurance plans, are improving access to cardiac treatments and encouraging earlier diagnosis and intervention
- The adoption of minimally invasive procedures, including TAVR, is gaining momentum, offering benefits such as reduced recovery times and lower risks for patients
- Medical tourism is on the rise, with patients from neighboring countries seeking affordable, high-quality valve replacement treatments in Asia-Pacific nations
Prosthetic Heart Valve Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- Medtronic (U.S.)
- Boston Scientific Corporation (U.S.)
- Edwards Lifesciences Corporation (U.S.)
- LivaNova PLC (U.K.)
- Cryolife, Inc. (U.S.)
- Lepu Medical Technology (Beijing) Co., Ltd. (China)
- Braile Biomédica (Brazil)
- SYMETIS (Switzerland)
- JenaValve (Germany)
- Colibri Heart Valve (U.S.)
- Cardiac Dimensions Inc. (U.S.)
- Cardiosolutions, Inc. (U.S.)
- Medtentia AB (Sweden)
- MitralSolutions, Inc. (U.S.)
- On-X Life Technologies, Inc. (U.S.)
- Sadra Medical, Inc. (U.S.)
- Sorin S.p.A. (Italy)
- St. Jude Medical, Inc. (U.S.)
- ValveXchange, Inc. (U.S.)
Latest Developments in Global Prosthetic Heart Valve Market
- In February 2024, Edwards Lifesciences Corporation introduced the EVOQUE tricuspid valve replacement system as the first transcatheter therapy approved by the U.S. Food and Drug Administration (FDA) for treating tricuspid regurgitation (TR). The system is indicated to improve health status in patients with severe symptomatic TR despite optimal medical therapy (OMT), when replacement of the tricuspid valve is considered appropriate by a heart team
- In March 2023, Abbott announced FDA approval for its Epic Max stented tissue valve to address aortic regurgitation or stenosis. The Epic Max features an optimized design aimed at enhancing blood flow through the valve, further solidifying its efficacy in treating patients with aortic valve disorders Prosthetic Heart Valve Market Scope
- In March 2022, Edwards Lifesciences received regulatory approval from the U.S. Food and Drug Administration for its MITRIS RESILIA valve. This newly approved device is a bioprosthetic (tissue) heart valve specifically engineered for use in the mitral position, a critical area for managing blood flow between the left atrium and left ventricle. The MITRIS RESILIA valve features advanced tissue preservation technology designed to improve durability and reduce calcification over time, making it a promising solution for patients requiring mitral valve replacement
- In January 2022, JenaValve Technology, Inc. formed a strategic partnership with Peijia Medical Limited, a leading medical device firm based in China, through a combination of equity investment and exclusive technology licensing. Under the terms of the agreement, Peijia made an initial financial investment and committed to ongoing support in exchange for exclusive development and commercialization rights to JenaValve’s Trilogy Transcatheter Aortic Valve Replacement (TAVR) system across the Greater China region. The Trilogy system is designed to treat patients with severe symptomatic aortic regurgitation or aortic stenosis—conditions that often require complex intervention
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.