Global Point Of Care Infectious Disease Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
18.17 Billion
USD
31.69 Billion
2024
2032
USD
18.17 Billion
USD
31.69 Billion
2024
2032
| 2025 –2032 | |
| USD 18.17 Billion | |
| USD 31.69 Billion | |
|
|
|
|
Globaler Markt für Point-of-Care-Infektionskrankheiten, nach technologischem Angebot (Molekulardiagnostik, Festphasen-, Lateral-Flow-, Agglutinationsassays und Durchfluss), klinischer Anwendung (Tropenkrankheiten, entzündliche Erkrankungen, Lebererkrankungen, HIV, Atemwegserkrankungen, HAIs, sexuell übertragbare Krankheiten und andere), Krankheiten (Hepatitis-B-Virus, Lungenentzündung/Streptokokken-assoziierte Infektionen, Grippe, Clostridium-difficile-Infektionen, Hepatitis-C-Virus, CDI, MRSA und TB), Endbenutzer (Krankenhäuser, Pflegeheime, häusliche Pflege, Kliniken, Diagnose- und Forschungslabore und andere) – Branchentrends und Prognose bis 2032.
Marktgröße für Point-of-Care-Infektionskrankheiten
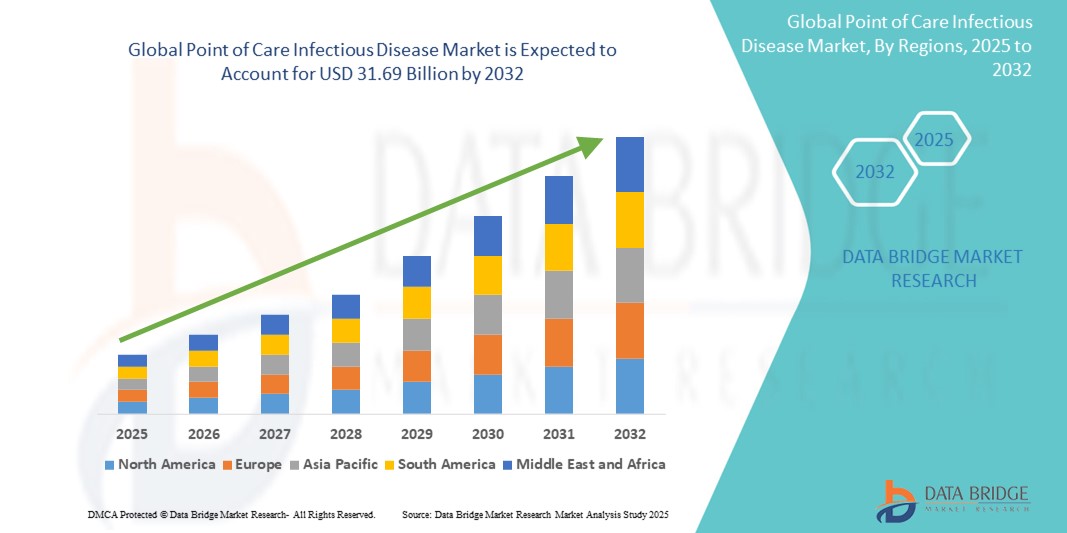
- Der globale Markt für Point-of-Care-Infektionskrankheiten hatte im Jahr 2024 ein Volumen von 18,17 Milliarden US-Dollar und dürfte bis 2032 einen Wert von 31,69 Milliarden US-Dollar erreichen , was einer jährlichen Wachstumsrate von 7,2 % im Prognosezeitraum entspricht.
- Das Marktwachstum wird maßgeblich durch die steigende Zahl von Infektionskrankheiten, die Nachfrage nach präzisen Diagnoseinstrumenten und technologische Fortschritte wie Molekulardiagnostik und Agglutinationstests vorangetrieben.
- Das zunehmende Bewusstsein der Verbraucher hinsichtlich der Frühdiagnose und rechtzeitigen Behandlung von Infektionskrankheiten treibt die Nachfrage nach Point-of-Care-Diagnostik von Infektionskrankheiten in verschiedenen Bereichen des Gesundheitswesens weiter voran.
Marktanalyse für Point-of-Care-Infektionskrankheiten
- Der Markt für Point-of-Care-Infektionskrankheiten verzeichnet ein stetiges Wachstum, da immer mehr Verbraucher und Gesundheitsdienstleister schnelle und genaue Diagnoselösungen für ein effizientes Krankheitsmanagement bevorzugen.
- Die steigende Nachfrage sowohl in Industrie- als auch in Entwicklungsländern ermutigt Hersteller, Innovationen mit leistungsstarken, tragbaren und benutzerfreundlichen Diagnoselösungen zu entwickeln.
- Nordamerika dominiert den Markt für Point-of-Care-Infektionskrankheiten mit dem größten Umsatzanteil von 51,5 % im Jahr 2024, getrieben durch eine steigende Zahl von Infektionskrankheiten und technologische Fortschritte, gepaart mit einer fortschrittlichen Gesundheitsinfrastruktur.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum die am schnellsten wachsende Region im Markt für Point-of-Care-Infektionskrankheiten sein. Dies ist auf den rasanten Anstieg der geriatrischen Bevölkerung, die steigende Zahl an Infektionskrankheiten und erhebliche Investitionen in die Verbesserung der Gesundheitsinfrastruktur zurückzuführen, insbesondere in Ländern wie China, Indien und den südostasiatischen Staaten.
- Das Segment der Molekulardiagnostik hält mit 43,5 % im Jahr 2024 den größten Marktanteil. Dies ist auf die überlegene Sensitivität und die Fähigkeit zurückzuführen, mehrere Krankheitserreger gleichzeitig zu identifizieren. Die Präzision und die schnellen Ergebnisse verbessern die Krankheitserkennung, unterstützen effiziente klinische Entscheidungen und fördern die weltweite Weiterentwicklung diagnostischer Technologien.
Berichtsumfang und Marktsegmentierung für Infektionskrankheiten am Behandlungsort
|
Eigenschaften |
Wichtige Markteinblicke zu Infektionskrankheiten am Behandlungsort |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen. |
Markttrends für Point-of-Care-Infektionskrankheiten
„Fortschritte in der Molekulardiagnostik und bei miniaturisierten Geräten“
- Der Markt für Point-of-Care-Infektionskrankheiten bevorzugt zunehmend molekulare Diagnosetechnologien aufgrund ihrer hohen Genauigkeit, Sensitivität und schnellen Durchlaufzeiten beim Nachweis von Krankheitserregern
- Miniaturisierte und tragbare Diagnosegeräte werden immer häufiger eingesetzt und ermöglichen Tests am oder in der Nähe des Patientenbetts, an entfernten Standorten und sogar für die häusliche Pflege.
- Diese Fortschritte ermöglichen eine schnelle Diagnose und einen sofortigen Behandlungsbeginn, was insbesondere bei der Bekämpfung von Infektionskrankheiten von entscheidender Bedeutung ist.
- So hat beispielsweise die Entwicklung von PCR-Schnelltests und Lab-on-a-Chip-Technologien die Möglichkeiten der POC-Tests auf Infektionskrankheiten erheblich erweitert.
- In Regionen mit hoher Krankheitslast wie Subsahara-Afrika werden molekulare POC-Tests bevorzugt, um Krankheiten wie HIV und TB zu erkennen und so die diagnostische Durchlaufzeit zu verkürzen.
- Kliniken und Krankenhäuser integrieren molekulare POC-Tests als Standard für das Management von Infektionskrankheiten
Marktdynamik für Point-of-Care-Infektionskrankheiten
Treiber
„Steigende Nachfrage nach schneller und zugänglicher Diagnostik“
- Das wachsende Bewusstsein für die Bedeutung einer frühen Diagnose von Infektionskrankheiten wie HIV, TB und Atemwegsinfektionen führt zu einer steigenden Nachfrage nach POC-Testlösungen
- POC-Tests reduzieren Diagnoseverzögerungen und ermöglichen eine schnellere Behandlung und bessere Patientenergebnisse, insbesondere in ressourcenbeschränkten Umgebungen.
- Diese Tests verbessern die Zugänglichkeit in abgelegenen Gebieten, reduzieren die Belastung zentraler Labore und verbessern das Krankheitsmanagement in Regionen wie Indien und Afrika.
- Gesundheitsdienstleister arbeiten mit Diagnostikunternehmen zusammen, um POC-Tests in die Routineversorgung zu integrieren
- Beispielsweise ermöglicht Abbotts ID NOW-Plattform schnelle molekulare Tests für Grippe und COVID-19 und verbessert so die diagnostische Effizienz
- Die zunehmende Resistenz gegen antimikrobielle Mittel, wie beispielsweise MRSA, treibt die Nachfrage nach Schnelldiagnostik zur gezielten Behandlung weiter an
Einschränkung/Herausforderung
„Herausforderungen im Bereich Regulierung und Erstattung“
- Strenge regulatorische Anforderungen für POC-Diagnosegeräte können den Markteintritt verzögern und die Entwicklungskosten für Hersteller erhöhen
- Unterschiedliche Erstattungsrichtlinien in den einzelnen Ländern erschweren die Marktexpansion, insbesondere bei teuren molekulardiagnostischen Tests
- Die eingeschränkte Sensitivität einiger POC-Tests im Vergleich zu Labormethoden kann in kritischen Fällen Bedenken hinsichtlich der Genauigkeit aufwerfen
- Beispielsweise werden einige Antigen-Schnelltests für Infektionskrankheiten auf falsch-negative Ergebnisse überprüft, was ihre Anwendung in bestimmten Umgebungen einschränkt.
- Diese Herausforderungen verhindern eine breite Akzeptanz und können das Marktwachstum in Regionen mit unterentwickelten Gesundheitssystemen einschränken.
Marktumfang für Point-of-Care-Infektionskrankheiten
Der Markt ist auf der Grundlage des technologischen Angebots, der klinischen Anwendung, der Krankheit und der Endbenutzer segmentiert.
- Nach Technologieangebot
Der globale Markt für Point-of-Care-Infektionskrankheiten ist auf Grundlage des technologischen Angebots in die Bereiche Molekulardiagnostik, Festphasendiagnostik, Lateral Flow, Agglutinationsassays und Durchflussdiagnostik unterteilt. Das Segment der Molekulardiagnostik hält 2024 mit 43,5 % den größten Marktanteil, was auf seine überlegene Sensitivität und die Fähigkeit zurückzuführen ist, mehrere Krankheitserreger gleichzeitig zu identifizieren. Seine Präzision und schnellen Ergebnisse verbessern die Krankheitserkennung, unterstützen effiziente klinische Entscheidungen und fördern die weltweite Weiterentwicklung diagnostischer Technologien.
Das Lateral-Flow-Segment wird voraussichtlich von 2025 bis 2032 mit 6,2 % die höchste Wachstumsrate verzeichnen. Dies ist auf seine Kosteneffizienz, sein benutzerfreundliches Design und die zunehmende Verbreitung in ressourcenarmen Umgebungen zurückzuführen. Die schnellen Ergebnisse und der minimale Infrastrukturbedarf treiben das Marktwachstum weiter voran.
- Nach klinischer Anwendung
Basierend auf der klinischen Anwendung ist der globale Markt für Point-of-Care-Infektionskrankheiten in Tropenkrankheiten, entzündliche Erkrankungen, Lebererkrankungen, HIV, Atemwegserkrankungen, nosokomialen Infektionen, sexuell übertragbare Krankheiten und weitere segmentiert. Das Segment Atemwegserkrankungen wird voraussichtlich 2025 den größten Marktanteil halten, vor allem aufgrund der hohen weltweiten Verbreitung von Atemwegsinfektionen wie Grippe, RSV und COVID-19- Diagnostik und der dringenden Notwendigkeit einer schnellen Diagnose zur Verhinderung einer weitverbreiteten Übertragung. Die Nachfrage nach sofortigen Ergebnissen in der Notfallversorgung und der Primärversorgung bei Erkrankungen wie Lungenentzündung und Bronchitis festigt seine führende Position weiter.
Das HIV-Segment wird voraussichtlich von 2025 bis 2032 die höchste jährliche Wachstumsrate verzeichnen. Dies ist auf globale Initiativen zur HIV-Frühdiagnose, verstärkte Aufklärungsprogramme und Fortschritte bei Point-of-Care-Testtechnologien zurückzuführen. Der Bedarf an zugänglichen Tests in abgelegenen Gebieten sowie die langfristigen Anforderungen an die HIV-Behandlung erfordern komfortable und schnelle Diagnoselösungen, die POC-Tests bieten. Die Ausweitung der Tests in der Schwangerschaftsvorsorge und bei Risikogruppen trägt ebenfalls maßgeblich zu diesem Wachstum bei.
- Durch Krankheit
Der globale Markt für Point-of-Care-Infektionskrankheiten ist nach Krankheitsbildern segmentiert in Hepatitis-B-Virus, Pneumonie/Streptokokken-assoziierte Infektionen, Influenza, Clostridium-difficile-Infektionen, Hepatitis-C-Virus, CDI, MRSA und TB. Das Influenza-Segment wird voraussichtlich 2025 den größten Marktanteil erwirtschaften. Dies ist auf die saisonalen Ausbrüche und die hohe globale Influenza-Inzidenz zurückzuführen, die flächendeckende und schnelle Tests erfordern, um die Reaktion des öffentlichen Gesundheitswesens zu unterstützen und rechtzeitig eine Behandlung einzuleiten. Die Verfügbarkeit wirksamer antiviraler Therapien unterstreicht die Notwendigkeit einer schnellen Diagnose zur Minimierung der Krankheitslast.
Das Tuberkulose-Segment (TB) wird voraussichtlich von 2025 bis 2032 die höchste durchschnittliche jährliche Wachstumsrate verzeichnen. Grund dafür sind die anhaltende globale Gesundheitsbelastung durch TB, insbesondere in Entwicklungsregionen, und der dringende Bedarf an schneller und präziser Diagnostik zur Eindämmung der Ausbreitung. Innovationen bei molekularen Point-of-Care-Tests zum Nachweis medikamentenresistenter TB-Stämme sind entscheidende Treiber für die weltweiten Bemühungen zur Eliminierung von TB und zur effektiven Patientenversorgung.
- Von Endbenutzern
Der globale Markt für Point-of-Care-Infektionskrankheiten ist nach Endnutzern in Krankenhäuser, Pflegeheime, häusliche Pflege, Kliniken, Diagnose- und Forschungslabore und weitere Bereiche unterteilt. Das Krankenhaussegment wird voraussichtlich im Jahr 2025 den größten Marktanteil halten, was auf die hohe Anzahl an Patientenaufnahmen, den Bedarf an schneller Diagnose in Notaufnahmen und die zunehmende Verbreitung von nosokomialen Infektionen zurückzuführen ist. Krankenhäuser dienen als zentrale Anlaufstellen für komplexe Diagnoseverfahren und verfügen über die Infrastruktur zur Integration verschiedener POC-Testplattformen.
Das Segment der häuslichen Pflege wird voraussichtlich von 2025 bis 2032 die höchste jährliche Wachstumsrate verzeichnen. Dies ist auf die zunehmende Nachfrage nach häuslicher Gesundheitsversorgung, die Weiterentwicklung benutzerfreundlicher POC-Geräte und die zunehmende Alterung der Bevölkerung zurückzuführen. Die Bequemlichkeit von Tests zu Hause, gepaart mit der Möglichkeit, chronische Leiden und Infektionskrankheiten aus der Ferne zu überwachen, machen die häusliche Pflege zu einem schnell wachsenden Segment. Die Entwicklung vernetzter Gesundheitsgeräte und der Telemedizin unterstützt dieses Wachstum zusätzlich.
Regionale Analyse des Point-of-Care-Marktes für Infektionskrankheiten
- Nordamerika dominiert den Markt für Point-of-Care-Infektionskrankheiten mit dem größten Umsatzanteil von 51,5 % im Jahr 2024, getrieben durch eine steigende Zahl von Infektionskrankheiten und technologische Fortschritte, gepaart mit einer fortschrittlichen Gesundheitsinfrastruktur.
- Verbraucher und Gesundheitsdienstleister bevorzugen POC-Tests, da sie eine schnellere Diagnose ermöglichen, eine rechtzeitige Behandlung ermöglichen und zur allgemeinen öffentlichen Gesundheit beitragen, insbesondere angesichts der hohen Inzidenz verschiedener Infektionskrankheiten.
- Das Wachstum wird durch Fortschritte in der Diagnosetechnologie und die zunehmende Akzeptanz sowohl im klinischen als auch im häuslichen Pflegebereich unterstützt.
Markteinblicke für Point-of-Care-Infektionskrankheiten in den USA
Es wird erwartet, dass die USA den nordamerikanischen Markt für Point-of-Care-Infektionskrankheiten mit dem höchsten Umsatzanteil dominieren werden. Dies wird durch die starke Nachfrage nach schnellen und präzisen Diagnoselösungen und das wachsende Bewusstsein der Verbraucher für die Vorteile einer frühzeitigen Krankheitserkennung begünstigt. Der Trend zu einer dezentralen Gesundheitsversorgung und die zunehmende regulatorische Unterstützung für Point-of-Care-Tests treiben das Marktwachstum weiter voran. Die zunehmende Integration fortschrittlicher Molekular- und Immunoassay-Technologien durch Hersteller unterstützt das Marktwachstum.
Markteinblicke für Infektionskrankheiten am Point-of-Care in Europa
Der europäische Markt für Point-of-Care-Infektionskrankheiten wird voraussichtlich deutlich wachsen, unterstützt durch den regulatorischen Schwerpunkt auf Krankheitsüberwachung und Strategien zur schnellen Intervention. Verbraucher und medizinisches Fachpersonal suchen nach Tests, die die diagnostischen Durchlaufzeiten verkürzen und gleichzeitig eine hohe Genauigkeit bieten. Das Wachstum ist sowohl im Krankenhaus- als auch im Primärversorgungsbereich deutlich ausgeprägt. Länder wie Deutschland und Frankreich verzeichnen aufgrund zunehmender Bedenken hinsichtlich der öffentlichen Gesundheit und des Bedarfs an effizientem Krankheitsmanagement eine deutliche Nachfrage.
Markteinblicke für Point-of-Care-Infektionskrankheiten in Großbritannien
Der britische Markt für Point-of-Care-Infektionskrankheiten wird voraussichtlich stark wachsen, angetrieben durch die Nachfrage nach verbesserter Diagnostik und schnellen Ergebnissen in städtischen und vorstädtischen Gebieten. Das zunehmende Interesse an öffentlichen Gesundheitsinitiativen und das wachsende Bewusstsein für die Vorteile der Früherkennung fördern die Akzeptanz. Darüber hinaus beeinflussen die Weiterentwicklung der Gesundheitspolitik und Investitionen in dezentrale Tests die Entscheidungen der Verbraucher und wägen Testgenauigkeit und Komfort ab.
Markteinblicke für Point-of-Care-Infektionskrankheiten in Deutschland
In Deutschland wird ein deutliches Wachstum der Point-of-Care-Diagnostik von Infektionskrankheiten erwartet. Dies ist auf die fortschrittliche Gesundheitsinfrastruktur und den hohen Verbraucherfokus auf effiziente und präzise Diagnostik zurückzuführen. Deutsche Gesundheitsdienstleister bevorzugen technologisch fortschrittliche Tests, die eine schnelle und zuverlässige Identifizierung von Krankheitserregern ermöglichen. Die Integration dieser Tests in Krankenhäuser, Kliniken und Notfalleinrichtungen unterstützt ein nachhaltiges Marktwachstum.
Markteinblicke für Point-of-Care-Infektionskrankheiten im asiatisch-pazifischen Raum
Der asiatisch-pazifische Raum dürfte das höchste Wachstum verzeichnen, angetrieben durch den Ausbau der Gesundheitsinfrastruktur und steigende verfügbare Einkommen in Ländern wie China, Indien und Japan. Das zunehmende Bewusstsein für Infektionsprävention, schnelle Diagnose und zugängliche Gesundheitslösungen treibt die Nachfrage an. Regierungsinitiativen zur Förderung der öffentlichen Gesundheit und der Krankheitsüberwachung fördern den Einsatz fortschrittlicher POC-Infektionstests zusätzlich.
Markteinblicke für Point-of-Care-Infektionskrankheiten in Japan
Der japanische Markt für Point-of-Care-Infektionskrankheiten dürfte aufgrund der starken Verbraucherpräferenz für hochwertige, technologisch fortschrittliche Diagnosetests, die die Patientenversorgung und die öffentliche Gesundheitssicherheit verbessern, stark wachsen. Die Präsenz großer Diagnostikhersteller und die Integration von POC-Tests in klinische Umgebungen beschleunigen die Marktdurchdringung. Das steigende Interesse an Früherkennung und personalisierter Medizin trägt ebenfalls zum Wachstum bei.
Markteinblicke für Point-of-Care-Infektionskrankheiten in China
China hält einen bedeutenden Anteil am Markt für Point-of-Care-Tests für Infektionskrankheiten im asiatisch-pazifischen Raum. Dies ist auf die rasante Urbanisierung, steigende Gesundheitsausgaben und die steigende Nachfrage nach schnellen und zugänglichen Diagnoselösungen zurückzuführen. Die wachsende Mittelschicht des Landes und der Fokus auf eine verbesserte Zugänglichkeit der Gesundheitsversorgung fördern die Einführung fortschrittlicher POC-Tests für Infektionskrankheiten. Starke inländische Produktionskapazitäten und wettbewerbsfähige Preise verbessern die Marktzugänglichkeit.
Marktanteile im Bereich Point-of-Care-Infektionskrankheiten
Die Point-of-Care-Branche für Infektionskrankheiten wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Abbott (USA)
- PTS Diagnostics (USA)
- F. Hoffmann-La Roche Ltd (Schweiz)
- Samsung Medison Co., Ltd. (Südkorea)
- Bio-Rad Laboratories, Inc. (USA)
- Die Menarini-Gruppe (Italien)
- Nova Biomedical (USA)
- AccuBioTech Co., Ltd. (China)
- BD (USA)
- Chembio Diagnostics, Inc. (USA)
- Danaher Corporation (USA)
- EKF DIAGNOSTICS HOLDINGS PLC (Großbritannien)
- Johnson & Johnson Services, Inc. (USA)
- QuidelOrtho Corporation (USA)
- Siemens (Deutschland)
Neueste Entwicklungen auf dem globalen Markt für Point-of-Care-Infektionskrankheiten
-
Im Januar 2025 brachte Abbott einen neuen Lateral-Flow-Test für den schnellen HIV-Nachweis auf den Markt, der sich auf ressourcenarme Regionen in Afrika und Asien konzentriert. Dieser hochempfindliche Test liefert schnelle und zuverlässige Ergebnisse und verbessert so die Frühdiagnose und den Zugang zu Behandlungen. Dank seiner Kosteneffizienz unterstützt er eine breite Akzeptanz in kommunalen Gesundheitsprogrammen. Die Innovation stärkt Abbotts Präsenz im globalen Point-of-Care-Markt (POC) und unterstreicht sein Engagement für erschwingliche und skalierbare Diagnostiklösungen.
- Im März 2024 erhielt bioMérieux die Zulassung der US-amerikanischen FDA und eine CLIA-Freistellung für sein BIOFIRE SPOTFIRE Respiratory/Sore Throat (R/ST) Panel und untermauerte damit seine führende Position in der Diagnostik von Atemwegsinfektionen. Dieser Multiplex-PCR-Test erkennt bis zu 15 gängige Erreger in etwa 15 Minuten und ermöglicht so schnelle und präzise Diagnosen. Die Zulassung stärkt die Wettbewerbsfähigkeit von bioMérieux und ermöglicht eine breitere klinische Anwendung in der Notfallversorgung, in Apotheken und im ambulanten Bereich. Durch die Optimierung der syndromischen Testung unterstützt das Panel den verantwortungsvollen Umgang mit Antibiotika und eine modernisierte Patientenversorgung.
- Im Januar 2024 übernahm Roche die Point-of-Care-Technologie von LumiraDx und integrierte deren fortschrittliche Immunoassay- und klinisch-chemische Plattform in sein Diagnostikportfolio. Diese Akquisition erweitert Roches Kompetenzen in der dezentralen Patientenversorgung und erweitert den Zugang zu schnellen und präzisen Tests in der Primärversorgung. Das Multi-Assay-System von LumiraDx konsolidiert verschiedene Tests auf einem einzigen Gerät, optimiert Arbeitsabläufe und verbessert die diagnostische Effizienz. Roche möchte diese Technologie nutzen, um molekulare Tests voranzutreiben und die globale Gesundheitsversorgung zu verbessern.
- Im Februar 2023 kooperierte Thermo Fisher Scientific mit Mylab Discovery Solutions, um in Indien hergestellte RT-PCR-Kits für die Diagnostik von Infektionskrankheiten einzuführen. Diese Zusammenarbeit verbessert die Zugänglichkeit und Erschwinglichkeit und zielt auf eine breitere Bevölkerung in Indien und weltweit ab. Die von CDSCO lizenzierten Kits ermöglichen eine schnelle und präzise Erkennung von Tuberkulose, Hepatitis, HIV und anderen Krankheiten. Durch den Einsatz fortschrittlicher molekularer Diagnostik zielt die Partnerschaft darauf ab, die Gesundheitsinfrastruktur zu stärken und die Ziele zur Eliminierung von Krankheiten zu erreichen.
- Im November 2022 führte LumiraDx Healthcare seinen hochsensitiven Point-of-Care-Antigentest für C-reaktives Protein (CRP) in ganz Indien ein, um antimikrobielle Resistenzen (AMR) zu bekämpfen. Dieses Schnelldiagnosetool liefert Ergebnisse innerhalb von vier Minuten anhand einer Blutprobe aus der Fingerkuppe und ermöglicht Ärzten so fundierte Entscheidungen über Antibiotika. Durch die Reduzierung unnötiger Verschreibungen unterstützt der Test AMR-Stewardship-Programme und verbessert die Patientenversorgung auf Intensivstationen, Kinderkliniken, Notaufnahmen und in Ambulanzen. Die Markteinführung entspricht den Prioritäten des indischen Gesundheitswesens und gewährleistet ein effektives Infektionsmanagement.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.