Global Pharmacogenetic Testing Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
610.34 Million
USD
1,346.87 Million
2024
2032
USD
610.34 Million
USD
1,346.87 Million
2024
2032
| 2025 –2032 | |
| USD 610.34 Million | |
| USD 1,346.87 Million | |
|
|
|
|
Global Pharmacogenetic Testing Market, By Type (Whole Genome Sequencing, Whole Exome Sequencing, Array-Based Tests, and Single Gene Tests), Gene Type (CYP2C19, CYP2D6, CYP2C9 and VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX and MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT, and Others), Drug Type (Prescription Drugs, Nutraceuticals, Recreational Drugs, Herbal Supplements, Vitamins, and Over-the-Counter Medications), Sample (Blood and Saliva), Therapeutic Area (Cardiology, Gastroenterology, Anesthesiology, Genomics, Endocrinology, Immunology & Hypersensitivity, Dermatology, Gynecology, Oncology, Neurology, and Others), Application (Clinical Practice, Drug Development, and Drug Regulation), End User (Healthcare Providers, Pharmaceutical & Biotechnology Companies, Research Centres and Academic Institutes, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacies, Mail-Order Pharmacies, and Direct-to-Customer Services) - Industry Trends and Forecast to 2030.
Pharmacogenetic Testing Market Analysis and Size
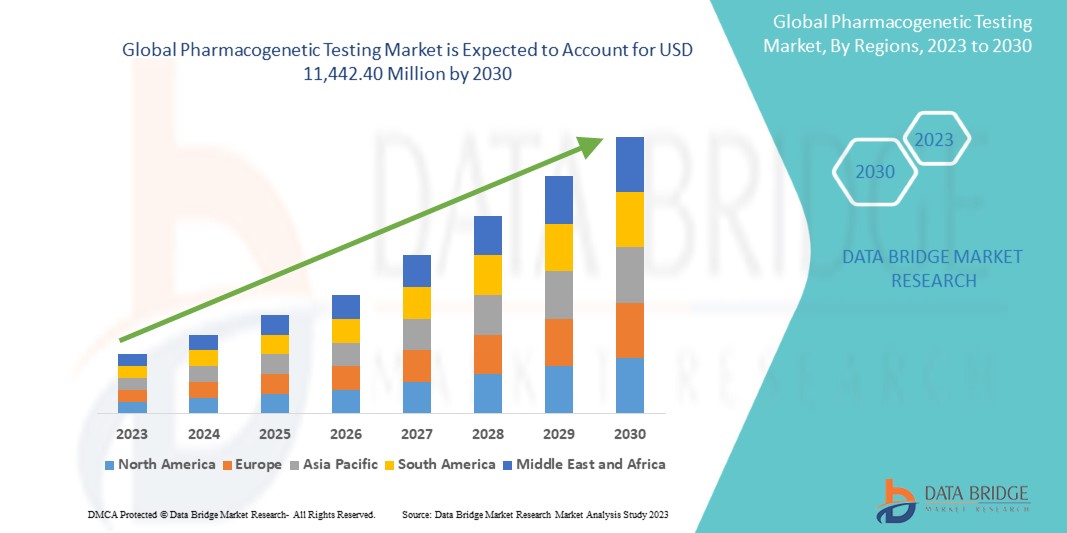
The global pharmacogenetic testing market is expected to gain market growth in the forecast period of 2023 to 2030. The market is fueled by the increasing adoption of personalized medicine which is a market that needed the pharmacogenetic testing market.
Integrating Electronic Health Records (EHRs) is a significant driver for the market growth. An increase in chronic and complex diseases is expected to create opportunities for market growth Data Bridge Market Research analyses that the market is growing with a CAGR of 8.7% in the forecast period of 2023 to 2030 and is expected to reach USD 11,442.40 million by 2030. Advancements in genomic technologies are expected to drive the market's expansion.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Year |
2021 (Customizable 2015-2020) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
Type (Whole Genome Sequencing, Whole Exome Sequencing, Array-Based Tests, and Single Gene Tests), Gene Type (CYP2C19, CYP2D6, CYP2C9 and VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX and MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT, and Others), Drug Type (Prescription Drugs, Nutraceuticals, Recreational Drugs, Herbal Supplements, Vitamins, and Over-the-Counter Medications), Sample (Blood and Saliva), Therapeutic Area (Cardiology, Gastroenterology, Anesthesiology, Genomics, Endocrinology, Immunology & Hypersensitivity, Dermatology, Gynecology, Oncology, Neurology, and Others), Application (Clinical Practice, Drug Development, and Drug Regulation), End User (Healthcare Providers, Pharmaceutical & Biotechnology Companies, Research Centres and Academic Institutes, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacies, Mail-Order Pharmacies, and Direct-to-Customer Services) |
|
Countries Covered |
U.S., Canada, Mexico, France, Germany, Spain, Italy, U.K., Spain, Netherlands, Russia, Turkey, Switzerland, Rest of Europe, China, Japan, Thailand, India, South Korea, Australia, Singapore, Indonesia, Philippines, Malaysia, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, U.A.E., Saudi Arabia, South Africa, Israel, Egypt, and Rest of Middle East and Africa |
|
Market Players Covered |
PerkinElmer Inc., Illumina, Inc., Thermo Fisher Scientific Inc., Dynamic DNA Laboratories, Sonic Healthcare, QIAGEN, Eurofins Scientific, 23andMe, Inc, OneOme, BGI, PacBio, MD Labs, GENEWIZ, PGXT, Luminex Corporation, and Myriad Genetics, Inc., among others |
Pharmacogenetic Testing Market Definition
Pharmacogenetic testing includes services that examine a person's genetic profile to ascertain how a person’s genes affect the reaction of the same to particular drugs. This specialized genetic testing identifies specific genetic variations that may affect different medications' metabolism, effectiveness, and potential side effects. Healthcare professionals can customize drug selections and dosages for a patient to improve treatment outcomes, reduce side effects, and increase overall drug efficacy. This is done by being aware of that patient's genetic predispositions. The quality of healthcare and patient outcomes may improve due to this personalized medication selection and dosage approach.
Pharmacogenetic Testing Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
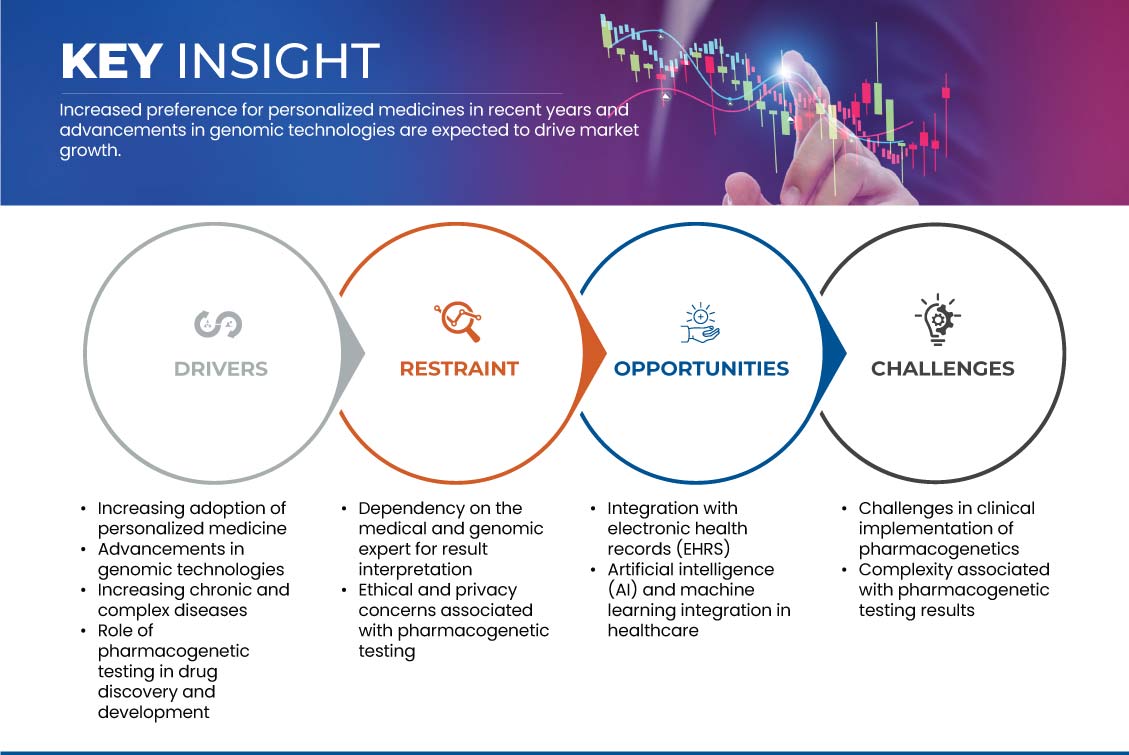
Drivers
- Increasing Adoption of Personalized Medicine
Pharmakogenetische Tests spielen eine entscheidende Rolle in der personalisierten Medizin, indem sie Arzneimittelbehandlungen an die genetische Ausstattung eines Individuums anpassen. Bei diesem innovativen Ansatz werden die genetischen Variationen eines Individuums analysiert, die sich auf den Stoffwechsel und die Reaktion auf bestimmte Medikamente auswirken können. Diese genetischen Unterschiede können die Wirksamkeit und möglichen Nebenwirkungen von Medikamenten beeinflussen, sodass pharmakogenetische Tests ein wertvolles Instrument für personalisierte Behandlungspläne sind.
Gesundheitsdienstleister können genetische Marker identifizieren, die bestimmen, wie der Körper Medikamente verarbeitet und nutzt, indem sie das individuelle genetische Profil einer Person untersuchen. Diese Informationen ermöglichen eine individuelle Auswahl und Dosierung von Medikamenten, um die Behandlungsergebnisse zu optimieren. Manche Personen können beispielsweise genetische Varianten aufweisen, die sie empfindlicher auf bestimmte Medikamente reagieren lassen, sodass geringere Dosen erforderlich sind, um die gewünschte therapeutische Wirkung ohne Nebenwirkungen zu erzielen. Andererseits können einige genetische Variationen höhere Dosen für dieselbe Wirkung erfordern. Dieser maßgeschneiderte Ansatz minimiert das „Versuch und Irrtum“, das oft mit der Medikamentenauswahl verbunden ist, verringert das Risiko von Nebenwirkungen und verbessert die Wirksamkeit der Behandlung. Er befähigt medizinisches Fachpersonal, fundierte Entscheidungen zu treffen und Medikamente zu verschreiben, die bei Patienten aufgrund ihrer genetischen Veranlagung wahrscheinlicher gut wirken. Letztendlich tragen pharmakogenetische Tests erheblich zum Paradigma der personalisierten Medizin bei und revolutionieren die Gesundheitsversorgung, indem sie präzisere und individuellere Behandlungsstrategien für bessere Patientenergebnisse fördern.
Der Markt profitiert also von diesem Nachfrageschub und erlebt einen stetigen Anstieg der F&E-Aktivitäten zur Entwicklung umfassenderer und präziserer Testlösungen. Technologische Fortschritte wie Next-Generation Sequencing (NGS) und andere molekulare Diagnosetechniken verbessern die Präzision und Erschwinglichkeit dieser Tests weiter. Das Ergebnis ist eine sich gegenseitig verstärkende Beziehung zwischen dem Aufstieg der personalisierten Medizin und dem florierenden Markt, wobei beide die Gesundheitslandschaft erheblich beeinflussen und eine Zukunft versprechen, in der Behandlungen maßgeschneidert, wirksam und patientenorientiert sind, was voraussichtlich das Marktwachstum vorantreiben wird.
- Fortschritte in der Genomtechnologie
Der anhaltende Aufschwung genomischer Innovationen hat die Präzision, Geschwindigkeit und Kosteneffizienz genetischer Tests deutlich verbessert. Next-Generation Sequencing (NGS) und andere hochmoderne molekulare Diagnosetechniken haben sich als entscheidende Werkzeuge herausgestellt und die Art und Weise, wie genetische Informationen analysiert und interpretiert werden, revolutioniert. Die Integration dieser fortschrittlichen genomischen Technologien hat die Genauigkeit und den Umfang pharmakogenetischer Tests verbessert und ermöglicht eine umfassende Bewertung des genetischen Profils einer Person. Dies wiederum ermöglicht es medizinischen Fachkräften, fundierte Entscheidungen hinsichtlich der Auswahl, Dosierung und Behandlungsstrategien zu treffen, die auf die einzigartige genetische Ausstattung eines Patienten zugeschnitten sind.
Darüber hinaus hat sich die CRISPR-Cas9-Genbearbeitungstechnologie bei der Untersuchung der funktionellen Auswirkungen bestimmter genetischer Varianten als vielversprechend erwiesen. Mithilfe dieser Technologie können Forscher Gene präzise modifizieren, bestimmte genetische Variationen nachahmen und ihre Auswirkungen auf den Arzneimittelstoffwechsel und die Arzneimittelwirkung aufklären. Diese Fortschritte tragen gemeinsam zur Verfeinerung und Ausweitung des Marktes bei und bringen eine Zukunft näher, in der Medikamente auf der Grundlage ihres einzigartigen genetischen Bauplans auf jeden Einzelnen zugeschnitten sind, was voraussichtlich das Marktwachstum vorantreiben wird.
Gelegenheit
- Integration mit elektronischen Gesundheitsakten (EHRs)
Die nahtlose Integration ermöglicht eine effiziente und zentralisierte Speicherung genetischer Daten neben umfassenden Patientenakten. Diese Zusammenführung verbessert die Zugänglichkeit und Nutzbarkeit pharmakogenetischer Informationen für medizinisches Fachpersonal und ermöglicht es ihnen, datengesteuerte Entscheidungen in Echtzeit zu treffen. Ärzte können bei der Behandlungsplanung problemlos auf genetische Erkenntnisse zugreifen und so sicherstellen, dass Medikamente verschrieben werden, die auf die individuelle genetische Ausstattung eines Patienten abgestimmt sind. Die durch diese Integration erzielten Effizienzgewinne beschleunigen die Einführung pharmakogenetischer Tests, tragen zu einer verbesserten Patientenversorgung, optimierten Arbeitsabläufen und letztlich zum Wachstum des Marktes bei.
Darüber hinaus erleichtert die Integration mit EHRs Forschung und Datenanalyse in größerem Maßstab. Aggregierte und anonymisierte genetische Daten in EHRs können für Forschung und klinische Studien genutzt werden und so Fortschritte in der Pharmakogenomik vorantreiben. Pharmaunternehmen, Forschungseinrichtungen und Gesundheitsdienstleister können diese Daten nutzen, um die Arzneimittelentwicklung zu verbessern, genetische Zusammenhänge zu validieren und Behandlungsstrategien zu optimieren. Diese Zusammenarbeit zwischen dem Markt und EHRs fördert Innovationen. Sie fördert ein synergetisches Ökosystem, das dem Gesundheitssektor zugutekommt und den Markt durch verbesserte Forschung, Produktentwicklung und datengesteuerte Entscheidungsfindung vorantreibt, was voraussichtlich Möglichkeiten für Marktwachstum schaffen wird.
Einschränkung/Herausforderung
- Abhängigkeit vom medizinischen und genomischen Experten bei der Ergebnisinterpretation
Der Markt ist mit einer erheblichen Einschränkung konfrontiert, da er bei der Interpretation der Ergebnisse stark auf medizinische und genomische Experten angewiesen ist. Die Komplexität genetischer Daten erfordert Spezialwissen und Fachwissen für ein genaues und aussagekräftiges Verständnis. Die korrekte Interpretation der Ergebnisse pharmakogenetischer Tests hängt in hohem Maße von hochqualifizierten Fachleuten ab, darunter Genetiker, Pharmakologen und Genomexperten. Diese Abhängigkeit stellt eine Herausforderung hinsichtlich der Skalierbarkeit und der weit verbreiteten Einführung pharmakogenetischer Tests dar. Der begrenzte Pool qualifizierter Experten und der Zeit- und Arbeitsaufwand für eine genaue Interpretation verzögern häufig die Bereitstellung umsetzbarer Erkenntnisse für medizinisches Personal und Patienten. Darüber hinaus kann diese Abhängigkeit von Experten zu höheren Kosten und einem höheren Ressourcenverbrauch führen und die nahtlose Integration pharmakogenetischer Tests in die klinische Routinepraxis behindern. Um diese Einschränkung zu überwinden, sind Fortschritte bei automatisierten Datenanalysetools, benutzerfreundlichen Schnittstellen und umfassenden Schulungsprogrammen erforderlich, um ein breiteres Spektrum an medizinischem Fachpersonal in die Lage zu versetzen, pharmakogenetische Testergebnisse genau zu interpretieren und zu nutzen. Daher bietet der Markt enormes Potenzial für die Anpassung von Arzneimitteltherapien an Einzelpersonen auf der Grundlage ihrer genetischen Ausstattung. Eine kritische Herausforderung besteht darin, dass man bei der Interpretation von Testergebnissen auf medizinische und genomische Experten angewiesen ist. Die Komplexität genetischer Daten erfordert spezielles Fachwissen, was die Interpretation der Ergebnisse und das Treffen klinischer Entscheidungen erschwert, was das Marktwachstum voraussichtlich bremsen wird.
Jüngste Entwicklung
- Im Mai 2023 kündigte PerkinElmer Inc. die Gründung von Revvity, Inc. als Anbieter wissenschaftlich fundierter Lösungen an, der Fortschritte in der Diagnostik und den Biowissenschaften nutzt, um das Leben der Menschen weltweit zu verbessern. Zuvor war das Unternehmen mit PerkinElmer, Inc. verbunden. Die Kunden von Revvity kommen aus verschiedenen Branchen, darunter Pharma und Biotechnologie, Diagnoselabore, Hochschulen und Regierungsorganisationen. Revvity bietet Reagenzien, Verbrauchsmaterialien, Assays, Instrumente und Software an
- Im Oktober 2022 gaben PacBio und Twist Bioscience Corporation die Verfügbarkeit eines ersten Portfolios handelsüblicher Genpanels mit langen Leselängen bekannt. Diese festen Twist Alliance-Panels sind darauf ausgelegt, Zielbereiche effizient und kostengünstig zu erfassen. Darüber hinaus können Kunden ein Panel ihres Designs erstellen, das für die Sequenzierung mit PacBio HiFi-Reads vollständig anpassbar und skalierbar ist.
Globaler Marktumfang für pharmakogenetische Tests
Der globale Markt für pharmakogenetische Tests ist in acht wichtige Segmente unterteilt, basierend auf Typ, Gentyp, Arzneimitteltyp, Probe, Therapiebereich, Anwendung, Endbenutzer und Vertriebskanal. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse wichtiger Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, um strategische Entscheidungen zur Identifizierung der wichtigsten Marktanwendungen zu treffen.
Typ
- Sequenzierung des gesamten Genoms
- Sequenzierung des gesamten Exoms
- Array-basierte Tests
- Einzelgentests
Auf der Grundlage des Typs ist der Markt in die Sequenzierung des gesamten Genoms, die Sequenzierung des gesamten Exoms, Array-basierte Tests und Einzelgentests segmentiert.
Gentyp
- CYP2C19
- CYP2D6
- CYP2C9 und VKORC1
- CYP1A2
- HLA-B*1502
- HLA-B*5701
- CYP2D
- OPRM1
- ONCOTYPE DX® und MAMMAPRINT®
- DRD3
- D4D4
- SLC6A4
- HTR2A/C
- TMPT
- Sonstiges
Auf der Grundlage des Gentyps ist der Markt in CYP2C19, CYP2D6, CYP2C9 und VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX und MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT und andere unterteilt.
Arzneimitteltyp
- Verschreibungspflichtige Medikamente
- Nutrazeutika
- Freizeitdrogen
- Pflanzliche Nahrungsergänzungsmittel
- Vitamine
- Frei verkäufliche Medikamente
Auf der Grundlage des Arzneimitteltyps ist der Markt in verschreibungspflichtige Arzneimittel, Nutrazeutika, Freizeitdrogen, pflanzliche Nahrungsergänzungsmittel, Vitamine und rezeptfreie Medikamente segmentiert.
Probe
- Blut
- Speichel
Auf der Grundlage der Probe wird der Markt in Blut und Speichel segmentiert.
Therapiebereich
- Kardiologie
- Gastroenterologie
- Anästhesie
- Genomik
- Endokrinologie
- Immunologie und Überempfindlichkeit
- Dermatologie
- Gynäkologie
- Onkologie
- Neurologie
- Sonstiges
Auf der Grundlage des Therapiebereichs ist der Markt in Kardiologie, Gastroenterologie, Anästhesiologie, Genomik, Endokrinologie, Immunologie und Überempfindlichkeit, Dermatologie, Gynäkologie, Onkologie, Neurologie und andere unterteilt.
Anwendung
- Klinische Praxis
- Arzneimittelentwicklung
- Arzneimittelregulierung
Auf der Grundlage der Anwendung ist der Markt in klinische Praxis, Arzneimittelentwicklung und Arzneimittelregulierung segmentiert.
Endbenutzer
- Gesundheitsdienstleister
- Pharma- und Biotechnologieunternehmen
- Zentren und akademische Institute
- Sonstiges
Auf der Grundlage des Endbenutzers wird der Markt in Gesundheitsdienstleister, Pharma- und Biotechnologieunternehmen, Forschungszentren und akademische Institute usw. segmentiert.
Vertriebskanal
- Krankenhausapotheke
- Einzelhandelsapotheken
- Versandapotheken
- Direkter Kundenservice
Auf der Grundlage der Vertriebskanäle ist der Markt in Krankenhausapotheken, Einzelhandelsapotheken, Versandapotheken und Direktkundendienste segmentiert.
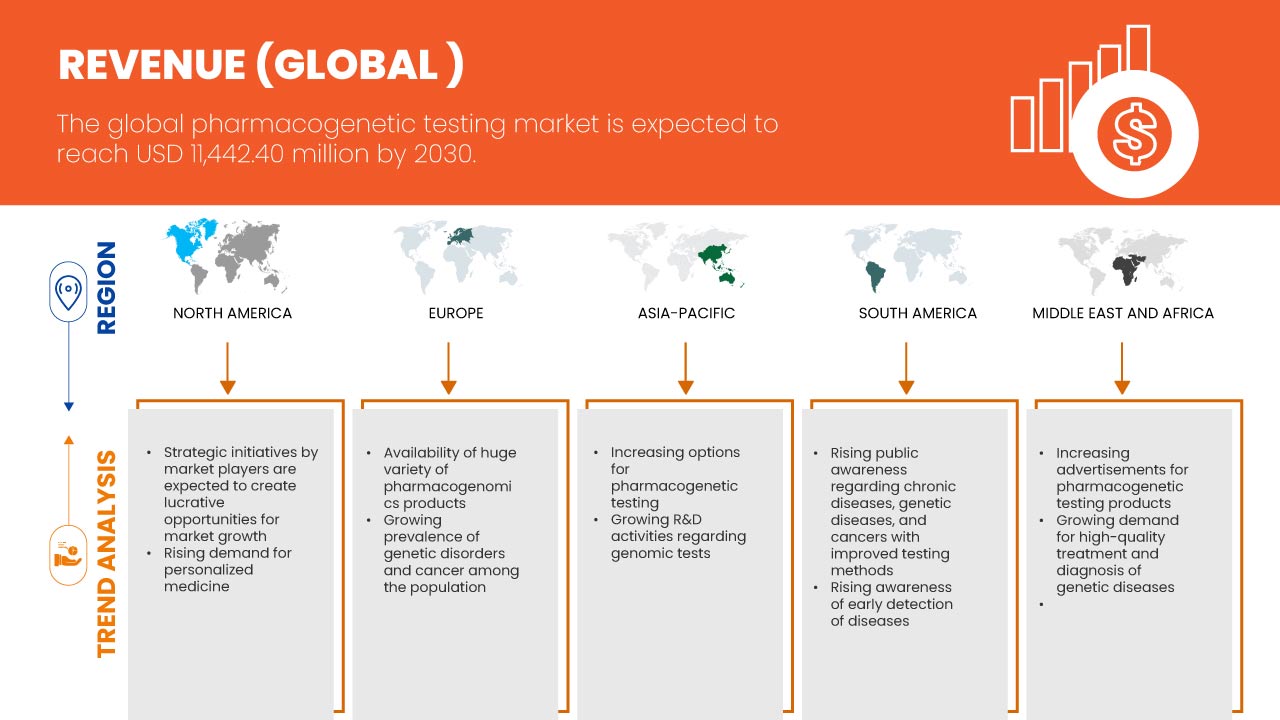
Regionale Analyse/Einblicke: Markt für pharmakogenetische Tests
Der globale Markt für pharmakogenetische Tests wird analysiert und Erkenntnisse sowie Trends zur Marktgröße werden auf der Grundlage von Typ, Gentyp, Arzneimitteltyp, Probe, Therapiebereich, Anwendung, Endnutzer und Vertriebskanal, wie oben angegeben, bereitgestellt.
Die im Marktbericht abgedeckten Länder sind die USA, Kanada, Mexiko, Frankreich, Deutschland, Spanien, Italien, Großbritannien, Spanien, Niederlande, Russland, Türkei, Schweiz, übriges Europa, China, Japan, Thailand, Indien, Südkorea, Australien, Singapur, Indonesien, Philippinen, Malaysia, übriger asiatisch-pazifischer Raum, Brasilien, Argentinien, übriges Südamerika, Vereinigte Arabische Emirate, Saudi-Arabien, Südafrika, Israel, Ägypten und der restliche Nahe Osten und Afrika.
Die USA werden voraussichtlich die Region Nordamerika dominieren, da sie über eine fortschrittliche Infrastruktur und ein ausgedehntes Netzwerk an Zentren für hochwertige Gesundheitsinfrastruktur verfügen. Deutschland wird voraussichtlich die Region Europa dominieren, da dort die Forschung und Entwicklung im Bereich Biotechnologie und Gesundheitswesen zunimmt. Japan wird voraussichtlich die Region Asien-Pazifik dominieren, da dort die Nachfrage steigt und das Bewusstsein für den Markt für pharmakogenetische Tests zunimmt.
Der Länderabschnitt des Berichts enthält auch individuelle marktbeeinflussende Faktoren und Änderungen der Marktregulierung, die die aktuellen und zukünftigen Trends des Marktes beeinflussen. Datenpunkte wie Downstream- und Upstream-Wertschöpfungskettenanalysen, technische Trends und Porters Fünf-Kräfte-Analyse sowie Fallstudien sind einige der Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Auch die Präsenz und Verfügbarkeit regionaler Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen inländischer Zölle und Handelsrouten werden bei der Bereitstellung von Prognoseanalysen der Länderdaten berücksichtigt.
Wettbewerbsumfeld und Marktanteilsanalyse für pharmakogenetische Tests
Die Wettbewerbslandschaft des globalen Marktes für pharmakogenetische Tests liefert Details nach Wettbewerbern. Zu den enthaltenen Details gehören Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, regionale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang, Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen auf den Markt.
Zu den wichtigsten Akteuren auf dem globalen Markt für pharmakogenetische Tests zählen unter anderem PerkinElmer Inc., Illumina, Inc., Thermo Fisher Scientific Inc., Dynamic DNA Laboratories, Sonic Healthcare, QIAGEN, Eurofins Scientific, 23andMe, Inc, OneOme, BGI, PacBio, MD Labs, GENEWIZ, PGXT, Luminex Corporation und Myriad Genetics, Inc.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.