Global Molecular Point Of Care Testing Using Naat Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
37.93 Billion
USD
86.17 Billion
2024
2032
USD
37.93 Billion
USD
86.17 Billion
2024
2032
| 2025 –2032 | |
| USD 37.93 Billion | |
| USD 86.17 Billion | |
|
|
|
|
Segmentierung des globalen Marktes für molekulare Point-of-Care-Tests (mittels NAAT), nachProdukt (Instrumente, Verbrauchsmaterialien und Reagenzien), Indikation (Tests auf Atemwegsinfektionen, sexuell übertragbare Infektionen (STI), Magen-Darm-Infektionen und Sonstige), Endnutzer (Labore, Krankenhäuser, Kliniken, ambulante Zentren, häusliche Pflege, Einrichtungen für betreutes Wohnen und Sonstige), Testart (verschreibungspflichtige und rezeptfreie Tests), Vertriebskanal (Krankenhausapotheke, Einzelhandelsapotheke und Online-Apotheke) – Branchentrends und Prognose bis 2032
Marktgröße für molekulare Point-of-Care-Tests (mittels NAAT)
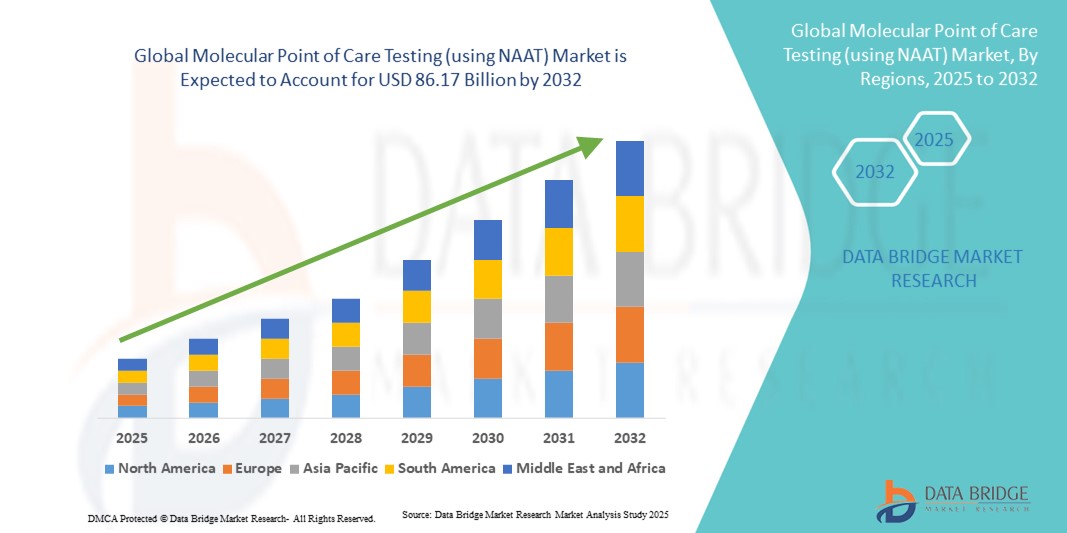
- Der globale Markt für molekulare Point-of-Care-Tests (mittels NAAT) hatte im Jahr 2024 einen Wert von 37,93 Milliarden US-Dollar und wird voraussichtlich bis 2032 auf 86,17 Milliarden US-Dollar anwachsen , was einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 1,08 % im Prognosezeitraum entspricht.
- Dieses Marktwachstum wird primär durch die zunehmende Verbreitung von Infektionskrankheiten , die Nachfrage nach schnellen und präzisen Diagnosetests sowie Fortschritte in der molekularen Diagnostik angetrieben.
- Darüber hinaus tragen der Trend zu einer dezentralen Gesundheitsversorgung und die zunehmende Nutzung von Multiplex-Tests zum Wachstum des Marktes für molekulare Point-of-Care-Tests bei. Diese Faktoren positionieren die molekulare Point-of-Care-Diagnostik als zentralen Bestandteil der modernen Diagnostik und ermöglichen die zeitnahe und präzise Erkennung verschiedener Erkrankungen.
Marktanalyse für molekulare Point-of-Care-Tests (mittels NAAT)
- Molekulare Point-of-Care-Tests (POCT) mittels Nukleinsäureamplifikationstests (NAAT) ermöglichen den schnellen und genauen Nachweis von Infektionskrankheiten und anderen Erkrankungen direkt am Patienten oder in dessen unmittelbarer Nähe und sind somit ein entscheidender Bestandteil der modernen Gesundheitsdiagnostik in Krankenhäusern, Kliniken und dezentralen Gesundheitseinrichtungen.
- Die zunehmende Verbreitung molekularer Point-of-Care-Tests (POCT) wird vor allem durch die steigende Prävalenz von Infektionskrankheiten, die wachsende Nachfrage nach zeitnaher und präziser Diagnostik sowie technologische Fortschritte bei tragbaren und benutzerfreundlichen molekularen Testgeräten vorangetrieben.
- Nordamerika dominierte den Markt für molekulare Point-of-Care-Tests (POCT) mit dem größten Umsatzanteil von 39 % im Jahr 2024. Dies wurde durch die frühe Einführung fortschrittlicher Diagnosetechnologien, hohe Gesundheitsausgaben und die starke Präsenz wichtiger Branchenakteure begünstigt. In den USA war das Wachstum aufgrund der Integration von Nukleinsäureamplifikationstests (NAAT) in Krankenhäuser, Kliniken und Notaufnahmen besonders ausgeprägt.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum die am schnellsten wachsende Region sein, angetrieben durch steigende Investitionen in die Gesundheitsinfrastruktur, ein zunehmendes Bewusstsein für schnelle Diagnoselösungen und eine wachsende Nachfrage nach patientennahen Tests in Schwellenländern.
- Das Segment der Tests auf Atemwegsinfektionen dominierte den Markt für molekulare Point-of-Care-Tests (POCT) mit einem Marktanteil von 43,2 % im Jahr 2024. Dies ist auf die hohe Prävalenz von infektiösen Atemwegserkrankungen und den klinischen Bedarf an einer schnellen und genauen Diagnose zurückzuführen, um eine rechtzeitige Behandlung und Eindämmungsmaßnahmen zu ermöglichen.
Berichtsumfang und Marktsegmentierung für molekulare Point-of-Care-Tests (mittels NAAT)
|
Attribute |
Molekulare Point-of-Care-Testung (mittels NAAT): Wichtigste Markteinblicke |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Mehrwertdaten-Infosets |
Zusätzlich zu Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und Hauptakteure enthalten die von Data Bridge Market Research erstellten Marktberichte auch detaillierte Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, einen Überblick über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und den regulatorischen Rahmen. |
Markttrends für molekulare Point-of-Care-Tests (mittels NAAT)
Fortschritte bei schnellen, multiplexen und KI-gestützten Tests
- Ein bedeutender und sich beschleunigender Trend auf dem globalen Markt für molekulare Point-of-Care-Tests (POCT) ist die Entwicklung schneller, multiplexfähiger Nukleinsäureamplifikationstests (NAAT) und KI-gestützter Diagnoseplattformen. Diese verbessern die Geschwindigkeit, Genauigkeit und Benutzerfreundlichkeit von Point-of-Care-Tests in Krankenhäusern, Kliniken und dezentralen Gesundheitseinrichtungen.
- Der Xpert Xpress SARS-CoV-2/Grippe/RSV-Test beispielsweise integriert den Nachweis mehrerer Krankheitserreger in einem einzigen Durchlauf, wodurch die Bearbeitungszeit verkürzt und der Arbeitsablauf am Patientenstandort optimiert wird.
- Die Integration von KI in die molekulare Point-of-Care-Diagnostik ermöglicht Funktionen wie die automatisierte Interpretation von Testergebnissen, prädiktive Analysen für Krankheitsausbrüche und intelligente Qualitätskontrolle, wodurch die diagnostische Sicherheit erhöht und menschliche Fehler reduziert werden.
- Diese KI-gestützten Plattformen ermöglichen es Gesundheitsdienstleistern, mehrere diagnostische Parameter gleichzeitig zu verwalten und so schnellere Ergebnisse bei kritischen Erkrankungen zu liefern, während gleichzeitig die Abhängigkeit von zentralen Laboren verringert wird.
- Dieser Trend hin zu schnelleren, präziseren und vernetzten Testlösungen verändert die Erwartungen an die patientennahe Diagnostik. Unternehmen wie Abbott und Roche entwickeln daher KI-gestützte NAAT-Geräte mit Multiplex-Funktionalität und benutzerfreundlichen Schnittstellen für das Klinikpersonal.
- Die Nachfrage nach molekularen POCT-Geräten mit Multiplex-Detektion und KI-gestützten Funktionen wächst sowohl im stationären als auch im ambulanten Bereich rasant, da Gesundheitsdienstleister zunehmend Wert auf schnelle und zuverlässige Diagnostik legen.
Marktdynamik der molekularen Point-of-Care-Diagnostik (mittels NAAT)
Treiber
Steigende Nachfrage aufgrund der Belastung durch Infektionskrankheiten und dezentraler Testverfahren
- Die weltweit zunehmende Verbreitung von Infektionskrankheiten, verbunden mit dem Trend zu einer dezentralen Gesundheitsversorgung, ist ein wesentlicher Faktor für die verstärkte Anwendung molekularer Point-of-Care-Tests (POCT) mittels Nukleinsäureamplifikationstests (NAAT).
- Beispielsweise hat Cepheid im Jahr 2024 erweiterte Anwendungsbereiche seiner GeneXpert-Plattform eingeführt, um auch sexuell übertragbare Infektionen und Atemwegserkrankungen zu untersuchen. Dies spiegelt die Bemühungen wider, dem wachsenden diagnostischen Bedarf direkt am Behandlungsort gerecht zu werden.
- Da Gesundheitsdienstleister eine schnellere Diagnose und zeitnahe Behandlung anstreben, bietet die NAAT-basierte patientennahe Sofortdiagnostik (POCT) eine hohe Sensitivität, Spezifität und schnelle Ergebnisse und stellt somit eine überzeugende Alternative zu herkömmlichen zentralen Labortests dar.
- Darüber hinaus führt die zunehmende Betonung des Ausbruchsmanagements und der Notfallvorsorge dazu, dass molekulare Point-of-Care-Tests (POCT) zu einem integralen Bestandteil von Programmen zur Bekämpfung von Infektionskrankheiten werden und schnelle Isolierungs- und Behandlungsentscheidungen ermöglichen.
- Die Bequemlichkeit patientennaher Tests, der minimale Aufwand bei der Probenverarbeitung und die Möglichkeit, mehrere Tests gleichzeitig durchzuführen, sind Schlüsselfaktoren, die die Einführung von NAAT-basierten molekularen Point-of-Care-Tests (POCT) in Krankenhäusern, Kliniken und im Außendienst vorantreiben.
Zurückhaltung/Herausforderung
Regulatorische Hürden und betriebliche Einschränkungen
- Die Einhaltung regulatorischer Vorgaben und strenge Zulassungsverfahren für molekulare POCT-Geräte stellen erhebliche Herausforderungen für die Marktexpansion dar, da NAAT-basierte Plattformen vor der Kommerzialisierung strenge Standards hinsichtlich Genauigkeit, Sicherheit und Zuverlässigkeit erfüllen müssen.
- Verzögerungen bei der FDA- oder CE-Zulassung können beispielsweise die Markteinführung innovativer POCT-Produkte verlangsamen und den Zugang zu fortschrittlichen Diagnostika in zeitkritischen Situationen einschränken.
- Betriebliche Einschränkungen wie der Bedarf an geschultem Personal, die Gerätewartung und die Versorgung mit Verbrauchsmaterialien können die Akzeptanz in ressourcenarmen Umgebungen trotz der technologischen Vorteile von NAAT-Geräten behindern.
- Zudem können die vergleichsweise hohen Kosten fortschrittlicher, multiplexfähiger oder KI-gestützter Point-of-Care-Testgeräte im Vergleich zu herkömmlichen Schnelltests für kleine Kliniken oder einkommensschwache Regionen ein Hindernis darstellen und deren flächendeckenden Einsatz einschränken.
- Obwohl die Kosten allmählich sinken, könnte der wahrgenommene Aufpreis für hochentwickelte molekulare POCT-Plattformen die Akzeptanz in budgetsensiblen Gesundheitseinrichtungen behindern.
- Die Bewältigung dieser Herausforderungen durch optimierte regulatorische Verfahren, Mitarbeiterschulungen und die Entwicklung kosteneffizienter Geräte wird für ein nachhaltiges Marktwachstum von entscheidender Bedeutung sein.
Marktumfang für molekulare Point-of-Care-Tests (mittels NAAT)
Der Markt ist segmentiert nach Produkt, Indikation, Endnutzer, Testmethode und Vertriebskanal.
- Nebenprodukt
Basierend auf dem Produkt ist der Markt für molekulare Point-of-Care-Diagnostik (POCT) in Geräte und Verbrauchsmaterialien & Reagenzien unterteilt. Das Segment der Geräte dominierte den Markt mit einem Umsatzanteil von 55,3 % im Jahr 2024. Dies ist auf die entscheidende Rolle automatisierter Nukleinsäureamplifikationstests (NAAT) zurückzuführen, die schnelle und präzise Ergebnisse liefern. Gesundheitseinrichtungen priorisieren die Einführung von Geräten aufgrund ihres hohen Durchsatzes, ihrer Zuverlässigkeit und ihrer Integration in Laborinformationssysteme . Investitionen in moderne Geräte ermöglichen es Krankenhäusern und Laboren, Arbeitsabläufe zu optimieren, die Effizienz zu steigern und die Bearbeitungszeiten für die Diagnostik von Infektionskrankheiten zu verkürzen. Das Segment profitiert von technologischen Innovationen wie tragbaren und kompakten Geräten, die sich für dezentrale Tests eignen. Geräte werden häufig mit Softwareplattformen kombiniert, um die automatisierte Berichterstellung und Qualitätskontrolle zu ermöglichen. Kontinuierlicher technischer Support und Wartungsdienste der Hersteller fördern die Akzeptanz zusätzlich.
Das Segment der Verbrauchsmaterialien und Reagenzien wird voraussichtlich von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 12,5 % das schnellste Wachstum verzeichnen. Treiber dieses Wachstums ist der wiederkehrende Bedarf an Kartuschen, Testkits und Reagenzien für NAAT-basierte Point-of-Care-Tests (POCT). Verbrauchsmaterialien sind für den täglichen Testbetrieb in Krankenhäusern, Kliniken und Laboren unerlässlich und generieren kontinuierliche Umsätze für die Anbieter. Das steigende Bewusstsein für Tests auf Infektionskrankheiten und die zunehmende Testfrequenz treiben die Nachfrage an. Multiplex-Assays, die spezielle Reagenzien erfordern, beschleunigen das Wachstum zusätzlich. Technologische Innovationen, die die Stabilität und Benutzerfreundlichkeit von Reagenzien verbessern, tragen zu deren Akzeptanz bei. Das Segment profitiert außerdem von Verbesserungen in der Lieferkette, die Lieferzeiten und -kosten reduzieren.
- Nach Indikation
Basierend auf den Indikationen ist der Markt in Tests für Atemwegsinfektionen, sexuell übertragbare Infektionen (STI), Magen-Darm-Infektionen und Sonstiges unterteilt. Das Segment der Atemwegsinfektionstests dominierte den Markt im Jahr 2024 mit einem Anteil von 43,2 %, bedingt durch die hohe Prävalenz von Krankheitserregern wie SARS-CoV-2, Influenza und RSV. Die schnelle und genaue Erkennung von Atemwegsinfektionen ist in Krankenhäusern, Kliniken und Notaufnahmen für eine zeitnahe Behandlung und Eindämmung unerlässlich. Nukleinsäureamplifikationstests (NAAT) auf Point-of-Care-Basis (POCT) bieten eine hohe Sensitivität und Spezifität und sind daher die bevorzugte Methode für Atemwegsinfektionen. Die Akzeptanz wird zusätzlich durch staatliche Programme zur Überwachung und Bekämpfung von Ausbrüchen gefördert. Krankenhäuser und Labore integrieren diese Tests in ihre Arbeitsabläufe in der Notfallversorgung, um schnelle Entscheidungen treffen zu können. Kontinuierliche Innovationen bei Multiplex-Panels erhöhen den Durchsatz und die Effizienz.
Der Markt für STI-Tests wird voraussichtlich von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 13,1 % am schnellsten wachsen. Treiber dieser Entwicklung sind das steigende Bewusstsein, Screening-Programme und die zunehmende Häufigkeit sexuell übertragbarer Infektionen. NAAT-basierte Point-of-Care-Tests (POCT) ermöglichen den schnellen, vertraulichen und präzisen Nachweis von STIs und eignen sich ideal für Kliniken und Heimtests. Multiplex-Assays, die mehrere STIs in einem einzigen Test nachweisen, beschleunigen die Akzeptanz. Die Ausweitung von Selbsttests und Initiativen zur sexuellen Gesundheit steigert die Nachfrage. Die Integration in Telemedizin-Plattformen verbessert die Ergebnisübermittlung und Beratung. Zunehmende Partnerschaften zwischen Diagnostikunternehmen und Gesundheitsdienstleistern fördern das Marktwachstum zusätzlich.
- Vom Endbenutzer
Basierend auf den Endnutzern ist der Markt für molekulare Point-of-Care-Tests (POCT) in Labore, Krankenhäuser, Kliniken, ambulante Zentren, häusliche Pflege, Einrichtungen für betreutes Wohnen und Sonstige unterteilt. Das Segment der Krankenhäuser dominierte den Markt mit dem größten Umsatzanteil von 48,6 % im Jahr 2024, bedingt durch das hohe Patientenaufkommen und den Bedarf an schneller Diagnostik. Krankenhäuser investieren in moderne Nukleinsäureamplifikationstests (NAAT), um Arbeitsabläufe zu optimieren, Infektionsausbrüche zu managen und die Notfallversorgung zu unterstützen. Die Integration in die Krankenhausinformationssysteme steigert die betriebliche Effizienz. Das Segment profitiert von kontinuierlichen technologischen Innovationen und staatlichen Förderprogrammen. Krankenhäuser benötigen häufig Geräte, die verschiedene Testarten durchführen können. Langfristige Verträge mit Lieferanten gewährleisten eine kontinuierliche Versorgung mit Geräten und Verbrauchsmaterialien.
Der Bereich der häuslichen Testung wird voraussichtlich von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 14,2 % das schnellste Wachstum verzeichnen. Treiber dieser Entwicklung sind die steigende Nachfrage nach Heimtests, deren Komfort und der Wunsch nach Datenschutz. Heimtests mit Nukleinsäureamplifikationstests (NAAT) ermöglichen es Patienten, sich testen zu lassen, ohne Kliniken oder Krankenhäuser aufsuchen zu müssen. Selbsttests auf Atemwegsinfektionen und sexuell übertragbare Infektionen (STI) fördern die Akzeptanz. Die Integration in Smartphone-Apps ermöglicht eine einfache Ergebnisinterpretation und telemedizinische Berichterstattung. Aufklärungskampagnen und Partnerschaften mit Telemedizinanbietern erweitern die Reichweite. Die zunehmende Akzeptanz von Heimtests durch die Gesundheitsbehörden unterstützt ein nachhaltiges Wachstum.
- Nach Testart
Basierend auf der Testmethode ist der Markt in verschreibungspflichtige und rezeptfreie (OTC-)Tests unterteilt. Verschreibungspflichtige Tests dominierten den Markt 2024 mit einem Anteil von 62,3 %, was auf regulatorische Anforderungen und die Aufsicht durch Fachkräfte zurückzuführen ist. Verschreibungspflichtige Tests bieten Genauigkeit, Zuverlässigkeit und klinische Interpretation, insbesondere in Krankenhäusern und Kliniken. Die Integration in die Arbeitsabläufe des Gesundheitswesens gewährleistet Patientensicherheit und Qualitätssicherung. Sie werden häufig bei Hochrisiko-Infektionskrankheiten eingesetzt, bei denen fachkundige Beratung erforderlich ist. Die Geräte sind oft mit Krankenhausnetzwerken verbunden, um Ergebnisse zu erfassen und zu melden. Die Hersteller verbessern kontinuierlich die Benutzerfreundlichkeit und erfüllen gleichzeitig die regulatorischen Standards.
Für den Markt für rezeptfreie Tests wird von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 13,5 % das schnellste Wachstum erwartet. Treiber dieser Entwicklung ist die steigende Nachfrage nach Selbsttests für Atemwegsinfektionen, sexuell übertragbare Infektionen und Magen-Darm-Infektionen. Benutzerfreundliche Kits und die Auswertung per Smartphone verbessern die Zugänglichkeit. Verbraucher bevorzugen rezeptfreie Lösungen aufgrund ihrer Bequemlichkeit, des Datenschutzes und der geringeren Anzahl an Arztbesuchen. Aufklärungskampagnen und die Integration von Telemedizin fördern die Akzeptanz. Einzelhändler und Online-Apotheken erhöhen die Verfügbarkeit. Der Markt profitiert von vereinfachten, vorverpackten Testkits für die Anwendung zu Hause.
- Nach Vertriebskanal
Basierend auf dem Vertriebskanal ist der Markt in Krankenhausapotheken, Einzelhandelsapotheken und Online-Apotheken unterteilt. Das Segment der Krankenhausapotheken dominierte den Markt mit einem Anteil von 57,4 % im Jahr 2024, bedingt durch die direkte Beschaffung für stationäre und ambulante Tests. Krankenhäuser nutzen Apothekenkanäle für eine kontinuierliche Versorgung mit NAAT-Geräten, Verbrauchsmaterialien und Reagenzien. Mengenrabatte senken die Kosten und gewährleisten eine rechtzeitige Wiederauffüllung. Die Integration in die Lieferkettensysteme der Krankenhäuser minimiert Fehlbestände. Krankenhäuser erhalten häufig technischen Support und Schulungen im Rahmen von Apothekenverträgen. Partnerschaften mit Herstellern stärken die Produktverfügbarkeit und den Service.
Für den Online-Apothekensektor wird von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 15,1 % das schnellste Wachstum erwartet. Treiber dieser Entwicklung sind die bequeme Bestellung, die Lieferung von NAAT-Kits nach Hause und die zunehmende Nutzung von Telemedizin. Online-Plattformen ermöglichen den diskreten Kauf von verschreibungspflichtigen und rezeptfreien Tests. Digitale Marketingkampagnen steigern die Bekanntheit bei den Verbrauchern. Die Integration mit Telemedizin-Apps ermöglicht die nahtlose Übermittlung von Testergebnissen. Verbraucher bevorzugen zunehmend den Online-Zugang aufgrund seiner Bequemlichkeit und des Datenschutzes. Das Wachstum des E-Commerce und die Verbesserungen in der Logistik unterstützen die rasche Expansion dieses Vertriebskanals.
Marktanalyse für molekulare Point-of-Care-Tests (mittels NAAT)
- Nordamerika dominierte den Markt für molekulare Point-of-Care-Tests (POCT) mit dem größten Umsatzanteil von 39 % im Jahr 2024. Dies wurde durch die frühe Einführung fortschrittlicher Diagnosetechnologien, hohe Gesundheitsausgaben und die starke Präsenz wichtiger Branchenakteure begünstigt. In den USA war das Wachstum aufgrund der Integration von Nukleinsäureamplifikationstests (NAAT) in Krankenhäuser, Kliniken und Notaufnahmen besonders ausgeprägt.
- Gesundheitsdienstleister in der Region legen Wert auf schnelle, genaue und zuverlässige Tests, weshalb NAAT-basierte Point-of-Care-Testgeräte (POCT) für Krankenhäuser, Kliniken und Notfallambulanzen unerlässlich sind. Insbesondere in den USA ist ein deutliches Wachstum zu verzeichnen, bedingt durch die Integration von NAAT-Geräten in Krankenhäuser, ambulante Einrichtungen und die häusliche Testung, unterstützt durch Innovationen etablierter Diagnostikunternehmen und Startups.
- Die weitverbreitete Akzeptanz wird zudem durch eine starke Gesundheitsinfrastruktur, hohe Gesundheitsausgaben und eine technologisch fortgeschrittene Bevölkerung begünstigt.
Markteinblicke zum US-amerikanischen Markt für molekulare Point-of-Care-Tests (mittels NAAT)
Der US-amerikanische Markt für molekulare Point-of-Care-Tests (POCT) erzielte 2024 mit 79 % den größten Umsatzanteil in Nordamerika. Treiber dieses Wachstums waren die rasche Verbreitung fortschrittlicher Diagnosetechnologien und der steigende Bedarf an zeitnaher Erkennung von Infektionskrankheiten. Gesundheitsdienstleister setzen aufgrund der hohen Genauigkeit und der schnellen Ergebnisse zunehmend auf Nukleinsäureamplifikationstests (NAAT) für Atemwegsinfektionen , sexuell übertragbare Infektionen und Magen-Darm-Infektionen. Der Trend zu dezentraler Gesundheitsversorgung und Heimtests beflügelt den Markt zusätzlich. Darüber hinaus trägt die Integration molekularer POCT-Geräte in Krankenhausinformationssysteme und Telemedizinplattformen maßgeblich zum Marktwachstum bei. Die starke Präsenz führender Diagnostikunternehmen und kontinuierliche technologische Innovationen unterstützen ebenfalls ein nachhaltiges Wachstum.
Markteinblicke für molekulare Point-of-Care-Tests in Europa (mittels NAAT).
Der europäische Markt für molekulare Point-of-Care-Tests (POCT) wird im Prognosezeitraum voraussichtlich ein deutliches Wachstum verzeichnen. Haupttreiber sind die steigende Nachfrage nach Schnelltests und die zunehmende Verbreitung von Infektionskrankheiten. Staatliche Initiativen zur Förderung von Point-of-Care-Tests und der Digitalisierung des Gesundheitswesens begünstigen deren Einsatz in Krankenhäusern, Kliniken und Laboren. Das Wachstum wird zudem durch die Urbanisierung und die steigende Prävalenz multiresistenter Infektionen unterstützt. Europäische Gesundheitsdienstleister setzen vermehrt auf Nukleinsäureamplifikationstests (NAAT) für ein zeitnahes Krankheitsmanagement und die Eindämmung von Ausbrüchen. Sowohl ambulante als auch stationäre Gesundheitseinrichtungen integrieren POCT-Geräte, um die Patientenversorgung zu verbessern. Der Markt verzeichnet insbesondere in Ländern wie Deutschland, Frankreich und Italien ein signifikantes Wachstum.
Markteinblicke für molekulare Point-of-Care-Tests in Großbritannien (mittels NAAT).
Der britische Markt für molekulare Point-of-Care-Tests (POCT) wird im Prognosezeitraum voraussichtlich ein beachtliches jährliches Wachstum verzeichnen. Treiber dieser Entwicklung ist die steigende Nachfrage nach Schnelltests und dezentralen Testlösungen. Das zunehmende Bewusstsein von Gesundheitsdienstleistern für die Vorteile von Nukleinsäureamplifikationstests (NAAT), wie z. B. hohe Sensitivität und Spezifität, fördert deren Einsatz in Krankenhäusern und Kliniken. Staatliche Initiativen zur Früherkennung und Behandlung von Infektionskrankheiten unterstützen die Marktexpansion zusätzlich. Die leistungsfähige Gesundheitsinfrastruktur und die robusten E-Health-Systeme des Landes verbessern die Geräteintegration und die Ergebnisübermittlung. Die zunehmende Nutzung im öffentlichen und privaten Gesundheitswesen stimuliert das Marktwachstum. Initiativen im Bereich Telemedizin und häusliche Pflege werden die Akzeptanz in Großbritannien voraussichtlich weiter steigern.
Markteinblicke für molekulare Point-of-Care-Tests in Deutschland (mittels NAAT).
Der deutsche Markt für molekulare Point-of-Care-Tests (POCT) wird im Prognosezeitraum voraussichtlich ein beachtliches Wachstum verzeichnen. Treiber dieser Entwicklung sind die zunehmende Verbreitung von Infektionskrankheiten und die steigende Nachfrage nach schnellen Diagnoseverfahren. Deutschlands fortschrittliche Gesundheitsinfrastruktur, der starke Fokus auf technologische Innovationen und das hohe Bewusstsein der medizinischen Fachkräfte fördern die Akzeptanz von NAAT-basierten POCT-Tests. Die Integration von POCT-Geräten in Krankenhausinformationssysteme und Labornetzwerke steigert die betriebliche Effizienz. Dezentrale Testverfahren in ambulanten Kliniken und Zentren gewinnen zunehmend an Bedeutung. Auch die Nutzung in der stationären Pflege und in kommerziellen Diagnoseeinrichtungen nimmt zu. Kontinuierliche Forschung und Entwicklung sowie die lokale Fertigung von POCT-Geräten und -Verbrauchsmaterialien stärken das Marktwachstum zusätzlich.
Markteinblicke für molekulare Point-of-Care-Tests (mittels NAAT) im asiatisch-pazifischen Raum
Der Markt für molekulare Point-of-Care-Tests (POCT) im asiatisch-pazifischen Raum wird im Prognosezeitraum von 2025 bis 2032 voraussichtlich mit einer jährlichen Wachstumsrate (CAGR) von 23,5 % am schnellsten wachsen. Treiber dieses Wachstums sind die steigende Inzidenz von Infektionskrankheiten, zunehmende Investitionen im Gesundheitswesen und der Ausbau der Testinfrastruktur in Ländern wie China, Japan und Indien. Der wachsende Fokus der Region auf dezentrale und häusliche Testlösungen fördert die Akzeptanz dieser Technologien. Staatliche Programme zur Förderung digitaler Gesundheitsversorgung und der Überwachung von Infektionskrankheiten unterstützen das Marktwachstum. Die Präsenz lokaler Hersteller gewährleistet erschwingliche Nukleinsäureamplifikationstests (NAAT) und Verbrauchsmaterialien. Die zunehmende Urbanisierung, die Nutzung neuer Technologien und das steigende Bewusstsein für die Bedeutung der Früherkennung tragen zusätzlich zum Wachstum bei. Die Nachfrage in Krankenhäusern, Kliniken und der häuslichen Pflege steigt stetig.
Markteinblicke für molekulare Point-of-Care-Tests in Japan (mittels NAAT).
Der japanische Markt für molekulare Point-of-Care-Tests (POCT) gewinnt aufgrund hoher Gesundheitsstandards, der zunehmenden Verbreitung fortschrittlicher Diagnosetechnologien und des wachsenden Bewusstseins für Schnelltests an Dynamik. Japan legt großen Wert auf die genaue und zeitnahe Erkennung von Infektionskrankheiten und fördert so die Anwendung in Krankenhäusern, Kliniken und der häuslichen Pflege. Die Integration von Nukleinsäureamplifikationstests (NAAT) in Krankenhausnetzwerke und Telemedizinplattformen steigert die Effizienz. Die alternde Bevölkerung erhöht die Nachfrage nach benutzerfreundlichen und leicht zugänglichen Testlösungen zusätzlich. Kontinuierliche Innovationen bei multiplexfähigen und tragbaren NAAT-Geräten unterstützen den Markt. Die wachsende Präferenz für Prävention und digitale Diagnostik beschleunigt die Einführung sowohl im privaten als auch im gewerblichen Gesundheitswesen.
Markteinblicke für molekulare Point-of-Care-Tests in Indien (mittels NAAT).
Der indische Markt für molekulare Point-of-Care-Tests (POCT) erzielte 2024 den größten Marktanteil im asiatisch-pazifischen Raum. Gründe hierfür sind die hohe Prävalenz von Infektionskrankheiten, die wachsende Mittelschicht und das steigende Gesundheitsbewusstsein in Indien. Die rasante Urbanisierung und staatliche Initiativen für intelligente Gesundheitsversorgung und digitale Diagnostik sind die wichtigsten Wachstumstreiber. Krankenhäuser, Kliniken und ambulante Pflegedienste setzen zunehmend auf NAAT-basierte POCT-Geräte für eine schnellere Diagnose und Behandlung. Erschwingliche Geräte lokaler Hersteller verbessern die Verfügbarkeit in städtischen und stadtnahen Gebieten. Die steigende Nachfrage nach Multiplex-Testlösungen beschleunigt die Markteinführung zusätzlich. Starke öffentliche Gesundheitsprogramme und Partnerschaften mit Diagnostikunternehmen fördern weiterhin das Marktwachstum.
Marktanteil der molekularen Point-of-Care-Diagnostik (mittels NAAT)
Die Branche der molekularen Point-of-Care-Tests (mittels NAAT) wird hauptsächlich von etablierten Unternehmen angeführt, darunter:
- Thermo Fisher Scientific Inc. (USA)
- Hologic, Inc. (USA)
- BD (USA)
- F. Hoffmann-La Roche AG (Schweiz)
- Abbott (USA)
- QIAGEN (Niederlande)
- BIOMÉRIEUX (Frankreich)
- Danaher (USA)
- Illumina, Inc. (USA)
- Sysmex Corporation (Japan)
- Siemens Healthineers AG (Deutschland)
- Seegene Inc. (Südkorea)
- Guardant Health, Inc. (USA)
- Labcorp (USA)
- Exact Sciences Corporation (USA)
- 10x Genomics, Inc. (USA)
- DNA Genotek Inc. (Kanada)
- PathoNostics (Niederlande)
- Molbio Diagnostics Limited (Indien).
Welche aktuellen Entwicklungen gibt es auf dem globalen Markt für molekulare Point-of-Care-Tests (mittels NAAT)?
- Im Juli 2025 erhielt BD (Becton, Dickinson and Company) die FDA-Zulassung 510(k) für sein BD Veritor™ System für SARS-CoV-2, einen digitalen Test zum Nachweis von COVID-19-Antigenen bei symptomatischen Personen in etwa 15 Minuten an Behandlungsorten wie Arztpraxen, Notfallambulanzen und Einzelhandelskliniken.
- Im Oktober 2024 genehmigte die Weltgesundheitsorganisation (WHO) den ersten Diagnosetest für Mpox (früher bekannt als Affenpocken) zur Notfallanwendung. Diese Zulassung soll den weltweiten Zugang zu schnellen und genauen Diagnoseverfahren für Mpox verbessern, insbesondere in ressourcenarmen Regionen. Der Test nutzt die Nukleinsäureamplifikationstechnologie und ermöglicht so eine patientennahe Diagnostik mit hoher Sensitivität und Spezifität.
- Im April 2024 brachte Roche Diagnostics das cobas® 5800 System auf den Markt, eine molekulardiagnostische Automatisierungsplattform der nächsten Generation, die die Produktivität steigern und Fehler in Laboren reduzieren soll. Das System bietet standardisierte Assays und skalierbare Lösungen und eignet sich daher für unterschiedliche Testvolumina und Probenmischungen. Durch die verbesserte Automatisierung in der molekularen Diagnostik optimiert das cobas® 5800 System Arbeitsabläufe und gewährleistet konsistente Ergebnisse in verschiedenen Testumgebungen.
- Im März 2023 gab die QuidelOrtho Corporation bekannt, dass ihr von der US-amerikanischen Arzneimittelbehörde FDA ein De-Novo-Antrag bewilligt wurde, der es dem Unternehmen ermöglicht, seinen neuen Schnelltest Sofia® 2 SARS Antigen+ FIA zu vermarkten. Der Sofia 2 SARS Antigen+ FIA ist der erste Schnelltest zum Nachweis von COVID-19, der die FDA-Zulassung erhalten hat.
- Im März 2023 wurde der LumiraDx SARS-CoV-2 Ag-Test für die Anwendung direkt am Behandlungsort in Einrichtungen zugelassen, die über eine CLIA-Zulassung (Certificate of Waiver, Certificate of Compliance oder Certificate of Accreditation) verfügen. Dieser Test ist für medizinisches Fachpersonal oder Anwender bestimmt, die mit der Durchführung von Tests direkt am Behandlungsort vertraut sind.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.