Global In Vitro Diagnostics Ivd Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
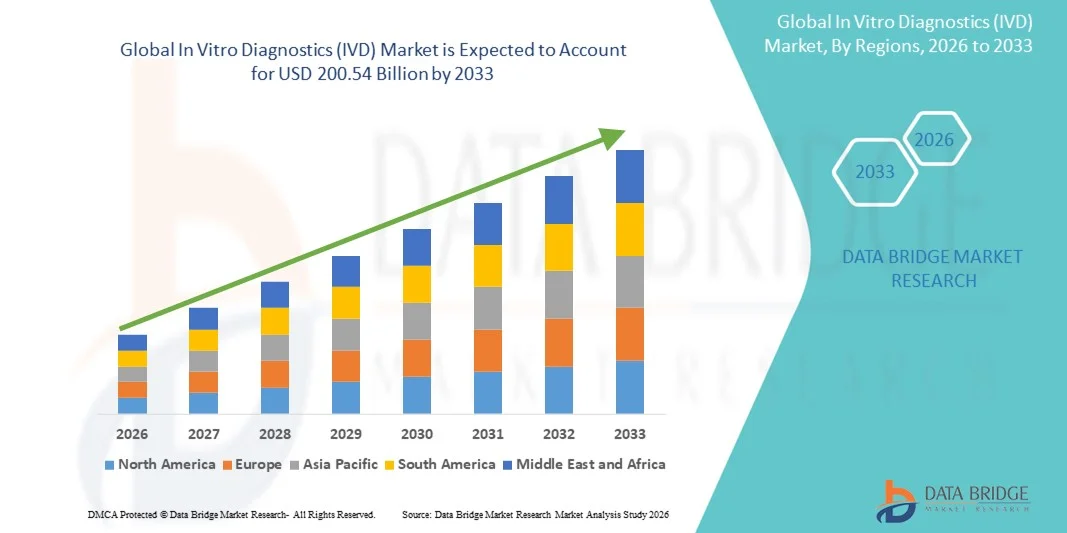
135.73 Billion
USD
200.54 Billion
2025
2033
USD
135.73 Billion
USD
200.54 Billion
2025
2033
| 2026 –2033 | |
| USD 135.73 Billion | |
| USD 200.54 Billion | |
|
|
|
|
Globale Marktsegmentierung für In-vitro-Diagnostika (IVD) nach Technik (Immunodiagnostik, Hämatologie, Molekulardiagnostik, Gewebediagnostik, In-vitro-Diagnostik (IVD), Sonstige), Anwendung (Infektionskrankheiten, Krebs, Herzerkrankungen, Erkrankungen des Immunsystems, Nephrologische Erkrankungen, Erkrankungen des Magen-Darm-Trakts, Sonstige), Endnutzer (Einzellaboratorien, Krankenhäuser, Universitäten und medizinische Fakultäten, Point-of-Care-Diagnostik, Sonstige), Produkt und Dienstleistung (Reagenzien, Instrumente, Software und Services) – Branchentrends und Prognose bis 2033
Marktgröße für In-vitro-Diagnostika (IVD)
- Der globale Markt für In-vitro-Diagnostika (IVD) hatte im Jahr 2025 einen Wert von 135,73 Milliarden US-Dollar und wird voraussichtlich bis 2033 auf 200,54 Milliarden US-Dollar anwachsen , was einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 5,00 % im Prognosezeitraum entspricht.
- Das Marktwachstum wird maßgeblich durch die zunehmende Verbreitung chronischer und infektiöser Krankheiten, die steigende Nachfrage nach frühzeitiger und präziser Krankheitserkennung sowie rasante technologische Fortschritte wie molekulare Diagnostik, patientennahe Sofortdiagnostik und Laborautomatisierung angetrieben.
- Darüber hinaus festigen der zunehmende Fokus auf personalisierte Medizin, der erweiterte Einsatz von Diagnostik in der Prävention und die wachsende Verbreitung KI-gestützter und integrierter Diagnoseplattformen die Position der In-vitro-Diagnostik (IVD) als unverzichtbaren Bestandteil der modernen Gesundheitsversorgung. Diese zusammenwirkenden Faktoren beschleunigen die Akzeptanz fortschrittlicher Diagnoselösungen und tragen somit maßgeblich zum Wachstum der Branche bei.
Marktanalyse für In-vitro-Diagnostika (IVD)
- In-vitro-Diagnostika (IVD), die diagnostische Tests an Blut, Gewebe und anderen biologischen Proben umfassen, sind aufgrund ihrer zentralen Rolle bei der Krankheitserkennung, -überwachung und personalisierten Behandlungsentscheidungen in Krankenhäusern, Laboren und bei Heimtests zunehmend unverzichtbare Bestandteile moderner Gesundheitssysteme.
- Die zunehmende Nachfrage nach IVD-Lösungen wird in erster Linie durch die weltweit steigende Verbreitung chronischer und infektiöser Krankheiten, den stärkeren Fokus auf frühzeitige und genaue Diagnosen sowie rasante technologische Fortschritte wie molekulare Diagnostik, patientennahe Tests, Automatisierung und KI-gestützte Plattformen angetrieben, die die Testgeschwindigkeit, Präzision und klinischen Ergebnisse verbessern.
- Nordamerika dominierte den Markt für In-vitro-Diagnostika (IVD) mit dem größten Umsatzanteil von 40,8 % im Jahr 2025. Dies wurde durch eine fortschrittliche Gesundheitsinfrastruktur, starke Erstattungssysteme, eine hohe Akzeptanz innovativer Diagnosetechnologien und die Präsenz führender globaler Hersteller begünstigt, die ihr Portfolio an molekularen und immunologischen Tests in den USA und Kanada kontinuierlich erweitern.
- Der asiatisch-pazifische Raum wird im Prognosezeitraum voraussichtlich die am schnellsten wachsende Region im Markt für In-vitro-Diagnostika (IVD) sein. Gründe hierfür sind steigende Gesundheitsausgaben, der Ausbau von Labornetzwerken, ein zunehmendes Bewusstsein für präventive Gesundheitsmaßnahmen und die wachsende Nachfrage nach zugänglichen und erschwinglichen Tests in dicht besiedelten Ländern wie China und Indien.
- Das Segment der Reagenzien dominierte 2025 den Markt für In-vitro-Diagnostika (IVD) mit einem Marktanteil von 65,50 %. Treiber dieser Entwicklung waren die stetig steigende Nachfrage nach Verbrauchsmaterialien, der zunehmende Einsatz molekularer und immunologischer Tests sowie die kontinuierlichen Fortschritte in der Assayentwicklung, die ein breites Spektrum diagnostischer Anwendungen in klinischen Bereichen unterstützen.
Berichtsgegenstand und Marktsegmentierung für In-vitro-Diagnostika (IVD)
|
Attribute |
In-vitro-Diagnostik (IVD): Wichtigste Markteinblicke |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Mehrwertdaten-Infosets |
Neben Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und Hauptakteure enthalten die von Data Bridge Market Research erstellten Marktberichte auch detaillierte Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und Informationen zum regulatorischen Rahmen. |
Markttrends für In-vitro-Diagnostika (IVD).
Verbesserte Diagnosegenauigkeit durch KI- und Automatisierungsintegration
- Ein bedeutender und sich beschleunigender Trend auf dem globalen Markt für In-vitro-Diagnostika (IVD) ist die zunehmende Integration von künstlicher Intelligenz ( KI), Automatisierung und digitaler Analytik in klinische Diagnosesysteme. Dies verbessert die Testgenauigkeit, Effizienz und das Workflow-Management in Krankenhäusern und Laboren erheblich.
- Beispielsweise integrieren automatisierte Immunoassay-Analysegeräte von Roche und Abbott nahtlos KI-gestützte Algorithmen, um die Probenverarbeitung zu optimieren, manuelle Fehler zu reduzieren und schnellere Bearbeitungszeiten für diagnostische Umgebungen mit hohem Probenaufkommen zu ermöglichen. Ebenso nutzen Plattformen wie Atellica von Siemens Healthineers intelligente Automatisierung für die präzise Probenhandhabung.
- Die Integration von KI in In-vitro-Diagnostikgeräte ermöglicht Funktionen wie vorausschauende Wartung, automatisierte Qualitätskontrolle und erweiterte Interpretation von Testergebnissen. So können KI-gestützte molekulare Systeme beispielsweise die Genauigkeit der Pathogendetektion verbessern und intelligente Warnmeldungen bei ungewöhnlichen Testmustern ausgeben, während automatisierte Arbeitsabläufe die Laborproduktivität deutlich steigern.
- Die nahtlose Integration von IVD-Analysegeräten in elektronische Patientenakten (EHRs) und digitale Diagnoseplattformen ermöglicht ein zentrales Datenmanagement und erlaubt Ärzten den Zugriff auf Patientenergebnisse zusammen mit Bildgebung, Behandlungsverlauf und Entscheidungshilfen – wodurch ein einheitliches diagnostisches Ökosystem entsteht.
- Dieser Trend hin zu intelligenteren, automatisierten und vernetzten Diagnosesystemen verändert grundlegend die Erwartungen an die Laborleistung. Unternehmen wie Sysmex und Beckman Coulter entwickeln daher fortschrittliche Analysegeräte mit KI-gestützten Entscheidungshilfen und Echtzeit-Überwachungsfunktionen für eine höhere betriebliche Effizienz.
- Die Nachfrage nach IVD-Systemen mit nahtloser Automatisierung, KI-gestützter Analytik und integrierter digitaler Vernetzung wächst in klinischen Laboren und patientennahen Einrichtungen rasant, da Gesundheitsdienstleister zunehmend Wert auf Genauigkeit, Geschwindigkeit und umfassende diagnostische Funktionalität legen.
Marktdynamik der In-vitro-Diagnostik (IVD)
Treiber
Wachsender Bedarf aufgrund zunehmender Krankheitslast und verstärkter Inanspruchnahme präventiver Gesundheitsmaßnahmen
- Die weltweit zunehmende Belastung durch chronische und Infektionskrankheiten, verbunden mit einem steigenden Fokus auf präventive Gesundheitsmaßnahmen, ist ein wesentlicher Treiber für die erhöhte Nachfrage auf dem Markt für In-vitro-Diagnostika (IVD).
- So kündigte Abbott beispielsweise im April 2025 Fortschritte bei seinen molekulardiagnostischen Plattformen mit verbesserten Hochdurchsatzkapazitäten zur schnelleren Pathogenerkennung an. Solche Entwicklungen wichtiger Akteure dürften das Wachstum der IVD-Branche im Prognosezeitraum vorantreiben.
- Da sich Gesundheitsdienstleister zunehmend der steigenden Krankheitsrisiken und der Notwendigkeit der Früherkennung bewusst werden, bieten IVD-Lösungen fortschrittliche Funktionen wie Echtzeitüberwachung, hochsensitives Screening und schnelle Diagnoseergebnisse und stellen damit einen entscheidenden Vorteil gegenüber herkömmlichen manuellen Testmethoden dar.
- Darüber hinaus macht die zunehmende Verbreitung digitaler Gesundheitssysteme und integrierter Diagnosenetzwerke IVD-Lösungen zu unverzichtbaren Bestandteilen moderner medizinischer Infrastruktur und bietet nahtlose Interoperabilität mit elektronischen Patientenakten und klinischen Entscheidungsplattformen.
- Die Bequemlichkeit schneller Tests, die Möglichkeit der Ferndiagnose und die verbesserte Verfügbarkeit durch patientennahe Geräte sind Schlüsselfaktoren für die zunehmende Verbreitung von In-vitro-Diagnostik (IVD) in Krankenhäusern, Kliniken und der häuslichen Pflege. Der Aufstieg dezentraler Testverfahren und benutzerfreundlicher molekularer und immunologischer Testgeräte trägt zusätzlich zum Marktwachstum bei.
Zurückhaltung/Herausforderung
Bedenken hinsichtlich der Datengenauigkeit und strenge regulatorische Hürden
- Bedenken hinsichtlich der Datengenauigkeit, der analytischen Variabilität und der Cybersicherheitslücken vernetzter Diagnosesysteme stellen erhebliche Herausforderungen für eine breitere Marktdurchdringung von In-vitro-Diagnostika dar. Da moderne Analysegeräte auf Software, Konnektivität und Automatisierung basieren, sind sie anfällig für Datenintegritätsrisiken und Systemverletzungen.
- Beispielsweise haben Berichte über Unstimmigkeiten bei bestimmten Schnelltests und Cybersicherheitslücken bei vernetzten Diagnosegeräten einige Gesundheitseinrichtungen vorsichtig bei der Einführung neuerer digitaler IVD-Plattformen gemacht.
- Um das Vertrauen der Nutzer zu gewinnen, ist es unerlässlich, diesen Bedenken durch robuste Datenschutzmaßnahmen, fortschrittliche Qualitätskontrollmechanismen und regelmäßige Softwareaktualisierungen zu begegnen. Unternehmen wie Roche und Siemens Healthineers betonen ihre strengen Compliance-Rahmenbedingungen und Sicherheitsstandards, um Käufer zu beruhigen. Darüber hinaus können die vergleichsweise hohen Kosten moderner IVD-Systeme im Vergleich zu traditionellen Labormethoden für Gesundheitseinrichtungen mit begrenztem Budget in Entwicklungsländern eine Hürde darstellen.
- Obwohl die Bezahlbarkeit durch kompakte, kostengünstige Analysegeräte verbessert wurde, sind Premium-Technologien wie Next-Generation-Sequenzierung (NGS), molekularbiologische Hochdurchsatzsysteme und automatisierte Immunoassay-Plattformen für viele Institutionen nach wie vor zu teuer, was ihre breite Anwendung einschränkt.
- Die Bewältigung dieser Herausforderungen durch eine verbesserte Einhaltung regulatorischer Vorgaben, erhöhte Systemsicherheit und die Entwicklung kostengünstigerer, hochpräziser Diagnoselösungen wird für eine nachhaltige Marktexpansion entscheidend sein.
Marktübersicht für In-vitro-Diagnostika (IVD)
Der Markt ist segmentiert nach Technik, Anwendung, Endnutzer sowie Produkt und Dienstleistung.
- Durch Technik
Basierend auf den angewandten Techniken ist der Markt für In-vitro-Diagnostik (IVD) in Immunodiagnostik, Hämatologie, Molekulardiagnostik, Gewebediagnostik und weitere Segmente unterteilt. Das Segment Immunodiagnostik dominierte den Markt mit dem größten Umsatzanteil im Jahr 2025, bedingt durch seine weitverbreitete Anwendung bei der Erkennung von Infektionskrankheiten, Autoimmunerkrankungen, Herz-Kreislauf-Erkrankungen und chronischen Krankheiten. Das Segment profitiert von hoher Testzuverlässigkeit, Automatisierungskompatibilität und kontinuierlichen Weiterentwicklungen in den Bereichen Chemilumineszenz, ELISA und Schnellimmunoassay. Immunodiagnostik bleibt zudem aufgrund ihrer Skalierbarkeit sowohl in Laboren mit hohem Probenaufkommen als auch in dezentralen Einrichtungen bevorzugt. Darüber hinaus stärken die hohe Nachfrage nach Antikörpertests und die zunehmende Anwendung in der patientennahen Diagnostik die führende Position des Segments im IVD-Bereich. Die steigende Prävalenz von Infektionskrankheiten und die zunehmende Verbreitung von Screening-Programmen tragen weiterhin maßgeblich zum starken Marktanteil dieses Segments bei.
Dem Segment der molekularen Diagnostik wird von 2026 bis 2033 das schnellste Wachstum prognostiziert. Treiber dieser Entwicklung ist die steigende Nachfrage nach Präzisionsmedizin, genomikbasierten Tests und der Früherkennung von Krankheiten. Die molekulare Diagnostik expandiert rasant aufgrund ihrer hohen Genauigkeit bei der Identifizierung von genetischem Material von Krankheitserregern und ihrer Bedeutung für die Onkologie, die Diagnostik von Infektionskrankheiten und die Auswahl personalisierter Therapien. Fortschritte bei PCR, Multiplex-Assays, NGS-Plattformen und molekularen Schnelltests beschleunigen ihre weltweite Anwendung. Der zunehmende Fokus auf die Früherkennung von Krebs und die Vorbereitung auf Krankheitsausbrüche stärkt dieses Segment zusätzlich. Darüber hinaus ermöglicht der Trend zu dezentralen Tests mittels tragbarer molekularer Geräte eine signifikante Verbreitung sowohl im klinischen als auch im nicht-klinischen Bereich.
- Durch Bewerbung
Basierend auf der Anwendung ist der Markt für In-vitro-Diagnostika (IVD) in Infektionskrankheiten, Krebs, Herzerkrankungen, Erkrankungen des Immunsystems, Nierenerkrankungen, Magen-Darm-Erkrankungen und Sonstiges unterteilt. Das Segment der Infektionskrankheiten dominierte den Markt mit dem größten Umsatzanteil im Jahr 2025, gestützt durch hohe weltweite Testvolumina für Atemwegsinfektionen, sexuell übertragbare Infektionen und Viruserkrankungen. Die starke Nachfrage nach Schnelltests, PCR-Assays und Antigen-basierten Kits hat die führende Position dieses Segments weiter gefestigt. Die zunehmende Häufigkeit neu auftretender und wiederkehrender Krankheitserreger treibt die routinemäßigen Screening- und Diagnoseaktivitäten in Krankenhäusern und Laboren weiter voran. Die Testung auf Infektionskrankheiten wird zudem durch ein wachsendes Bewusstsein, staatliche Überwachungsprogramme und die zunehmende Verfügbarkeit dezentraler Testlösungen ausgebaut. Das Segment profitiert von starken Innovationen sowohl bei Schnelltesttechnologien als auch bei automatisierten Laborsystemen.
Im Krebssegment wird von 2026 bis 2033 das schnellste Wachstum erwartet, getrieben durch die zunehmende Nutzung genetischer und biomarkerbasierter Tests zur Früherkennung und Therapieplanung. Der weltweite Anstieg der Krebsprävalenz verstärkt den Bedarf an präzisen und sensitiven Diagnoseverfahren entlang der gesamten onkologischen Behandlungskette. Flüssigbiopsien, Tumormarkertests und molekulare Onkologieplattformen gewinnen stark an Bedeutung. Fortschritte in der Genomprofilierung und der Begleitdiagnostik ermöglichen personalisierte Therapieentscheidungen und beschleunigen so das Wachstum dieses Segments. Steigende Investitionen in Krebs-Screening-Programme, gepaart mit technologischen Fortschritten in der minimalinvasiven Diagnostik, unterstützen die rasche Expansion dieses Segments zusätzlich.
- Vom Endbenutzer
Basierend auf den Endnutzern ist der Markt für In-vitro-Diagnostik (IVD) in eigenständige Labore, Krankenhäuser, Universitäten und medizinische Fakultäten, patientennahe Labore und Sonstige unterteilt. Das Segment der eigenständigen Labore dominierte den Markt mit dem größten Umsatzanteil im Jahr 2025. Gründe hierfür waren hohe Testvolumina, eine leistungsstarke Infrastruktur für komplexe Diagnostik sowie die breite Anwendung von Automatisierung und digitalen Arbeitsabläufen. Eigenständige Labore fungieren häufig als zentrale Anlaufstellen für spezialisierte Tests und ermöglichen durch moderne Geräte schnellere Bearbeitungszeiten und höhere Genauigkeit. Ihre Fähigkeit, große Probenmengen zu verarbeiten, positioniert sie als wichtige Anbieter nationaler und regionaler Diagnostikdienstleistungen. Darüber hinaus stärken verstärkte Partnerschaften mit Gesundheitsdienstleistern und der Ausbau von Diagnosenetzwerken die führende Position dieses Segments. Das Wachstum im Bereich der präventiven Gesundheitsvorsorge und der Überwachung chronischer Erkrankungen steigert die Nachfrage nach Tests in eigenständigen Laboren zusätzlich.
Das Segment der patientennahen Diagnostik (Point-of-Care-Diagnostik, POC) wird voraussichtlich von 2026 bis 2033 das schnellste Wachstum verzeichnen. Treiber dieser Entwicklung ist die steigende Nachfrage nach schnellen, mobilen und dezentralen Testlösungen im klinischen und nicht-klinischen Bereich. POC-Geräte ermöglichen Entscheidungen in Echtzeit und somit eine schnellere Diagnose und einen rascheren Behandlungsbeginn. Der zunehmende Einsatz kompakter Analysegeräte, Antigen-Schnelltests, Blutzuckermessgeräte und molekularer POC-Plattformen trägt zur Verbreitung bei. Das Segment profitiert von Fortschritten in den Bereichen Vernetzung und Miniaturisierung, wodurch Tests auch in ländlichen, abgelegenen Gebieten und in der häuslichen Pflege zugänglicher werden. Die steigende Nachfrage nach Komfort, die geringere Abhängigkeit von zentralen Laboren und die wachsenden Anwendungsbereiche in der Infektionskrankheitsbehandlung und im chronischen Krankheitsmanagement treiben das Wachstum des Segments weiterhin an.
- Nach Produkt und Dienstleistung
Basierend auf Produkten und Dienstleistungen ist der Markt für In-vitro-Diagnostika (IVD) in Reagenzien, Instrumente sowie Software und Services unterteilt. Das Reagenziensegment dominierte den Markt mit einem Umsatzanteil von 65,50 % im Jahr 2025. Dies ist auf die kontinuierliche Nachfrage nach Verbrauchsmaterialien für den täglichen Laborbetrieb und klinische Tests zurückzuführen. Reagenzien bilden das Rückgrat der IVD-Prozesse und ermöglichen deren kontinuierliche Anwendung in Immunoassays, der Molekulardiagnostik, der Hämatologie und der Biochemie. Das Segment profitiert von steigenden Testvolumina, der zunehmenden Verbreitung chronischer Erkrankungen und regelmäßigen Beschaffungszyklen von Laboren und Krankenhäusern. Fortschritte in der Assay-Chemie und die verstärkte Nutzung hochempfindlicher Reagenzien sichern die anhaltende Marktführerschaft. Darüber hinaus festigen die häufigen Markteinführungen krankheitsspezifischer Testkits die Position der Reagenzien als umsatzstärkste Produktkategorie.
Das Segment Software & Services wird voraussichtlich von 2026 bis 2033 das schnellste Wachstum verzeichnen. Treiber dieser Entwicklung ist die zunehmende Integration digitaler Diagnostik, Laborinformationssysteme (LIS) und Datenanalyseplattformen. Das Segment expandiert rasant aufgrund des Bedarfs an verbesserter Workflow-Automatisierung, optimierter Interoperabilität und Echtzeit-Datenmanagement in diagnostischen Umgebungen. Die wachsende Nutzung KI-gestützter Interpretationstools und cloudbasierter Diagnoseplattformen verstärkt dieses Wachstumspotenzial zusätzlich. Auch der zunehmende Fokus auf patientenzentrierte Diagnostik und Fernüberwachung trägt zur Nachfrage nach digitalen, servicebasierten Lösungen bei. Darüber hinaus verbessert softwaregestützte Automatisierung die Laboreffizienz, reduziert Fehler und ermöglicht schnellere klinische Entscheidungen.
Regionale Analyse des Marktes für In-vitro-Diagnostika (IVD).
- Nordamerika dominierte den Markt für In-vitro-Diagnostika (IVD) mit dem größten Umsatzanteil von 40,8 % im Jahr 2025. Dies wurde durch eine fortschrittliche Gesundheitsinfrastruktur, starke Erstattungssysteme, eine hohe Akzeptanz innovativer Diagnosetechnologien und die Präsenz führender globaler Hersteller begünstigt, die ihr Portfolio an molekularen und immunologischen Tests in den USA und Kanada kontinuierlich erweitern.
- Verbraucher und Gesundheitsdienstleister in der Region legen Wert auf präzise, schnelle und zuverlässige Diagnoselösungen, was zu einer starken Nachfrage nach molekularer Diagnostik, Immunoassays und patientennahen Testverfahren führt. Der starke Fokus auf Früherkennung, Prävention und personalisierte Medizin festigt weiterhin die Marktführerschaft Nordamerikas.
- Diese Vormachtstellung wird zusätzlich durch günstige Erstattungspolitiken, ein hohes Bewusstsein für routinemäßige Gesundheitsvorsorgeuntersuchungen und kontinuierliche technologische Innovationen gestützt, wodurch sich IVD-Lösungen als unverzichtbare Instrumente für die klinische Entscheidungsfindung und das Management chronischer Erkrankungen im öffentlichen und privaten Gesundheitswesen etablieren.
Einblick in den US-amerikanischen Markt für In-vitro-Diagnostika (IVD).
Der US-amerikanische Markt für In-vitro-Diagnostika (IVD) erzielte 2025 den größten Umsatzanteil in Nordamerika. Treiber dieser Entwicklung sind das hohe Testvolumen, die starke Verbreitung fortschrittlicher Labortechnologien und eine gut ausgebaute Gesundheitsinfrastruktur. Die steigende Nachfrage nach Früherkennung, Begleitdiagnostik und routinemäßigen Vorsorgeuntersuchungen treibt das Marktwachstum weiter an. Die zunehmende Verbreitung chronischer und infektiöser Krankheiten hat den Einsatz molekularer Diagnostik, Immunoassays und patientennaher Laborgeräte in klinischen Einrichtungen beschleunigt. Der wachsende Fokus auf personalisierte Medizin und schnelle Behandlungsentscheidungen stärkt die Rolle von IVD-Lösungen auf dem US-Markt. Darüber hinaus tragen kontinuierliche Innovationen inländischer Hersteller und die Integration digitaler Diagnoseplattformen maßgeblich zum Marktwachstum bei.
Einblick in den europäischen Markt für In-vitro-Diagnostika (IVD).
Der europäische Markt für In-vitro-Diagnostika (IVD) wird im Prognosezeitraum voraussichtlich ein deutliches Wachstum verzeichnen. Begünstigt wird dieses Wachstum durch strenge regulatorische Standards, ein hohes diagnostisches Bewusstsein und die breite Anwendung technologisch fortschrittlicher Testmethoden. Steigende Gesundheitsausgaben in den großen europäischen Volkswirtschaften treiben die Einführung innovativer Diagnoseplattformen in Krankenhäusern und Laboren voran. Der zunehmende Fokus der Region auf Prävention und die wachsende Zahl älterer Menschen erhöhen die Nachfrage nach häufigen diagnostischen Tests. Europäische Verbraucher und Gesundheitsdienstleister bevorzugen hochpräzise, automatisierte und standardisierte Diagnosesysteme. Darüber hinaus verstärkt das Wachstum von Screening-Programmen für Krebs, Herz-Kreislauf-Erkrankungen und Infektionskrankheiten die Akzeptanz von IVD in verschiedenen klinischen Bereichen.
Einblick in den britischen Markt für In-vitro-Diagnostika (IVD).
Der britische Markt für In-vitro-Diagnostika (IVD) wird im Prognosezeitraum voraussichtlich ein beachtliches jährliches Wachstum verzeichnen. Treiber dieser Entwicklung sind der steigende Bedarf an Diagnostik, die zunehmende Belastung durch chronische Krankheiten und die wachsende Nachfrage nach innovativen Testlösungen. Der starke Fokus des Landes auf Prävention und Früherkennung beschleunigt weiterhin die Einführung fortschrittlicher IVD-Technologien. Die zunehmende Bedeutung digitaler Gesundheitslösungen und die Integration von Laborinformationssystemen verbessern die diagnostische Effizienz in medizinischen Einrichtungen. Investitionen in die Modernisierung der Gesundheitsinfrastruktur unterstützen den Ausbau molekularer Tests und patientennaher Diagnostik. Darüber hinaus stimuliert das wachsende Bewusstsein für Screening-Programme und Krankheitsüberwachung das Wachstum des IVD-Marktes in Großbritannien zusätzlich.
Einblick in den deutschen Markt für In-vitro-Diagnostika (IVD).
Der deutsche Markt für In-vitro-Diagnostik (IVD) wird im Prognosezeitraum voraussichtlich ein beachtliches Wachstum verzeichnen. Treiber dieser Entwicklung sind hohe Investitionen in Medizintechnik und eine starke Nachfrage nach zuverlässigen Diagnosesystemen. Deutschlands fortschrittliche Laborinfrastruktur und der Fokus auf Präzisionsdiagnostik fördern weiterhin die breite Akzeptanz automatisierter Plattformen und molekularer Diagnostik. Der hohe Stellenwert von Qualität, Datensicherheit und regulatorischer Konformität in Deutschland entspricht dem steigenden Bedarf an hochentwickelten Diagnoselösungen. Die zunehmende Verbreitung chronischer Erkrankungen und die wachsende Nachfrage nach personalisierter Therapieplanung unterstützen das kontinuierliche Wachstum des IVD-Sektors. Auch die Integration innovativer Technologien, einschließlich KI-gestützter Diagnostik, gewinnt in deutschen Gesundheitseinrichtungen immer mehr an Bedeutung.
Markteinblicke für In-vitro-Diagnostika (IVD) im asiatisch-pazifischen Raum
Der Markt für In-vitro-Diagnostika (IVD) im asiatisch-pazifischen Raum wird im Prognosezeitraum voraussichtlich die höchste durchschnittliche jährliche Wachstumsrate (CAGR) aufweisen. Treiber dieser Entwicklung sind die großen Bevölkerungszahlen, die rasante Urbanisierung und das steigende Gesundheitsbewusstsein in Ländern wie China, Japan und Indien. Die zunehmende Häufigkeit von Infektionskrankheiten und chronischen Erkrankungen beschleunigt den Einsatz von Diagnosetests erheblich. Staatliche Initiativen zur Förderung der Digitalisierung des Gesundheitswesens und zum Ausbau der Laborinfrastruktur tragen zur verstärkten Akzeptanz von IVD in der gesamten Region bei. Darüber hinaus erhöht die Rolle des asiatisch-pazifischen Raums als globaler Produktionsstandort für Diagnostik-Kits, Reagenzien und Instrumente die Erschwinglichkeit und Verfügbarkeit für die Verbraucher vor Ort. Die wachsende Nachfrage nach patientennahen Tests und Schnelltests stärkt den Wachstumskurs der Region weiterhin.
Einblick in den japanischen Markt für In-vitro-Diagnostika (IVD).
Der japanische Markt für In-vitro-Diagnostik (IVD) gewinnt aufgrund des technologisch fortschrittlichen Gesundheitswesens und der steigenden Nachfrage nach hochpräzisen Diagnoseverfahren zunehmend an Dynamik. Die alternde Bevölkerung Japans treibt die verstärkte Nutzung von Diagnoselösungen für das Management chronischer Erkrankungen und die Vorsorgeuntersuchungen voran. Der Einsatz molekularer Diagnostik, automatisierter Analysegeräte und biomarkerbasierter Plattformen nimmt in Krankenhäusern und Laboren stetig zu. Die Integration von IVD-Systemen in umfassendere digitale Gesundheitsökosysteme, einschließlich elektronischer Patientenakten und KI-gestützter Plattformen, beflügelt das Wachstum weiterhin. Darüber hinaus verstärkt der Fokus des Landes auf Innovation, Effizienz und Früherkennung von Krankheiten die zunehmende Verbreitung modernster Diagnosetechnologien.
Einblick in den indischen Markt für In-vitro-Diagnostik (IVD).
Der indische Markt für In-vitro-Diagnostik (IVD) wird 2025 einen der größten Marktanteile im asiatisch-pazifischen Raum erzielen. Gründe hierfür sind die rasante Urbanisierung, der verbesserte Zugang zur Gesundheitsversorgung und das steigende Bewusstsein für präventive Diagnostik. Die wachsende Mittelschicht und die zunehmende Belastung durch Infektions- und chronische Krankheiten treiben die starke Nachfrage nach erschwinglichen und präzisen Testlösungen an. Indien entwickelt sich aufgrund steigender Investitionen in Krankenhäuser und expandierender privater Diagnosenetzwerke zu einem dynamischen Markt für laborbasierte und patientennahe Diagnostik. Staatliche Initiativen wie die „Digital Health Mission“ und erhöhte Gesundheitsausgaben beschleunigen das Marktwachstum zusätzlich. Die Präsenz starker inländischer Hersteller und die Verfügbarkeit kostengünstiger Testkits fördern weiterhin die breite Akzeptanz von IVD in städtischen und ländlichen Gebieten.
Marktanteil der In-vitro-Diagnostik (IVD)
Die In-vitro-Diagnostikbranche (IVD) wird hauptsächlich von etablierten Unternehmen dominiert, darunter:
- F. Hoffmann-La Roche AG (Schweiz)
- Abbott (USA)
- Thermo Fisher Scientific Inc. (USA)
- Siemens Healthineers AG (Deutschland)
- Danaher (USA)
- BD. (USA)
- BIOMÉRIEUX (Frankreich)
- Bio-Rad Laboratories (USA)
- Hologic, Inc. (USA)
- QIAGEN (Niederlande)
- PerkinElmer (USA)
- DiaSorin SpA (Italien)
- Luminex Corporation (USA)
- QuidelOrtho Corporation (USA)
- Nova Biomedical Corporation (USA)
- Meso Scale Diagnostics, LLC (USA)
- Ortho Clinical Diagnostics (USA)
- Sysmex Corporation (Japan)
- Seegene Inc. (Südkorea)
Welche aktuellen Entwicklungen gibt es auf dem globalen Markt für In-vitro-Diagnostika (IVD)?
- Im Januar 2024 schlug die Europäische Kommission eine Verlängerung der Übergangsfristen für Hersteller gemäß der Verordnung über In-vitro-Diagnostika (IVDR) vor. Ziel der Aktualisierung ist es, Engpässe bei wichtigen Diagnosetests zu vermeiden, indem Unternehmen mehr Zeit zur Einhaltung der strengeren regulatorischen Anforderungen eingeräumt wird. Zudem wird die EUDAMED-Datenbank schrittweise eingeführt, um Transparenz und Marktüberwachung zu stärken.
- Im April 2023 ging Thermo Fisher Scientific eine Partnerschaft mit ALPCO-GeneProof ein, um sein Portfolio an CE-IVD-zertifizierten molekulardiagnostischen Tests zu erweitern. Im Rahmen dieser Zusammenarbeit wurden 37 PCR-basierte Assays unter der Produktlinie TaqPath Menu | GeneProof eingeführt, die Infektionskrankheiten wie sexuell übertragbare Infektionen, respiratorische Viren und gastrointestinale Erreger abdecken. Dies erweiterte das Testangebot für Diagnostiklabore, die bereits PCR-Systeme von Thermo Fisher einsetzen, erheblich.
- Im Januar 2023 brachte QIAGEN das EZ2 Connect MDx auf den Markt, ein automatisiertes, CE-IVD-zertifiziertes System zur DNA/RNA-Extraktion. Die Plattform kann Nukleinsäuren aus bis zu 24 Proben gleichzeitig in ca. 30 Minuten aufreinigen und steigert so die Laboreffizienz. Durch die Automatisierung werden manuelle Arbeitsschritte reduziert, das Kontaminationsrisiko minimiert und die Reproduzierbarkeit verbessert.
- Im Juni 2021 brachte Thermo Fisher Scientific das TaqPath COVID-19 Fast PCR Combo Kit 2.0 auf den Markt, einen CE-IVD-zertifizierten Diagnosetest. Das Kit weist SARS-CoV-2 direkt in Speichelproben nach und macht so aufwendige Extraktionsschritte überflüssig. Es liefert Ergebnisse in weniger als zwei Stunden und ermöglicht dadurch häufige Tests in Krankenhäusern, Flughäfen und Betrieben.
- Der Test nutzt mehrere genetische Zielstrukturen, um die Genauigkeit auch bei der Entstehung neuer Varianten zu gewährleisten.
- Im März 2021 stellte QIAGEN den QIAcube Connect MDx vor, einen CE-IVD-zertifizierten Automaten für die standardisierte Probenvorbereitung. Das Gerät ermöglicht es Diagnostiklaboren, eine Vielzahl von IVD-zugelassenen Protokollen mit hoher Reproduzierbarkeit durchzuführen. Es unterstützt die vollständige Automatisierung von Aufreinigungsprozessen für DNA, RNA und Proteine. Dies reduziert die Variabilität zwischen verschiedenen Laboranten und gewährleistet eine strenge Qualitätskontrolle in klinischen Testumgebungen.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.