Global Herceptin Biosimilars Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1,810.00 Million
USD
11,302.00 Million
2022
2030
USD
1,810.00 Million
USD
11,302.00 Million
2022
2030
| 2023 –2030 | |
| USD 1,810.00 Million | |
| USD 11,302.00 Million | |
|
|
|
|
Globaler Markt für Herceptin-Biosimilars, nach Anwendung (Brustkrebs, Dickdarmkrebs, Leukämie, Lymphom und andere), Dosierung (150 mg/Einzeldosisfläschchen und 420 mg/Mehrfachdosisfläschchen), Endbenutzer (Fachkliniken, Krankenhäuser, Onkologiezentren und andere), Vertriebskanal (Direktausschreibung, Krankenhausapotheke, Einzelhandelsapotheke, Online-Apotheke und andere) – Branchentrends und Prognose bis 2030.
Herceptin-Biosimilars-Marktanalyse und -größe
Die Inzidenz der chronischen lymphatischen Leukämie (CLL) liegt in den USA bei etwa 21.000 Neuerkrankungen pro Jahr. Zum Vergleich: Die geschätzte Inzidenz des Non-Hodgkin-Lymphoms (NHL) liegt in den USA bei etwa 77.000 Neuerkrankungen pro Jahr. Die Inzidenz der akuten lymphatischen Leukämie (ALL) ist mit geschätzten 6.000 Neuerkrankungen pro Jahr in den Vereinigten Staaten viel geringer.
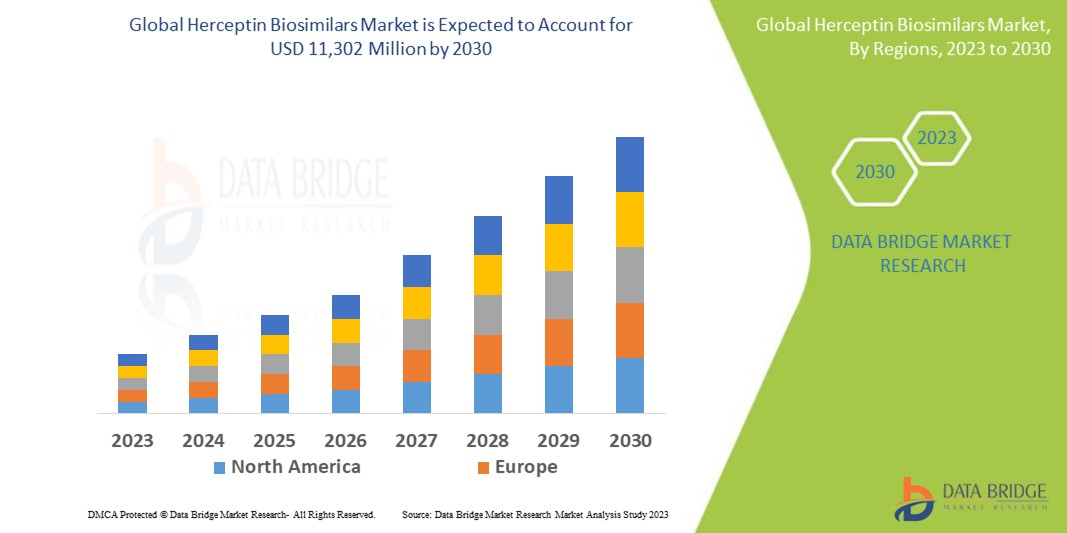
Data Bridge Market Research analysiert, dass der Markt, der im Jahr 2022 1.810 Millionen USD betrug, bis 2030 auf 11.302 Millionen USD anwachsen und im Prognosezeitraum eine durchschnittliche jährliche Wachstumsrate von 23,2 % aufweisen wird. Dies zeigt den Marktwert. „Brustkrebs“ dominiert das Anwendungssegment des Herceptin-Biosimilars-Marktes aufgrund der weltweit zunehmenden Verbreitung von Brustkrebs. Neben Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research zusammengestellten Marktberichte auch eingehende Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
Herceptin-Biosimilars – Marktumfang und -segmentierung
|
Berichtsmetrik |
Details |
|
Prognosezeitraum |
2023 bis 2030 |
|
Basisjahr |
2022 |
|
Historische Jahre |
2021 (anpassbar auf 2015–2020) |
|
Quantitative Einheiten |
Umsatz in Millionen USD, Mengen in Einheiten und Preise in USD |
|
Abgedeckte Segmente |
Nach Anwendung (Brustkrebs, Dickdarmkrebs, Leukämie, Lymphom und andere), Dosierung (150 mg/Einzeldosisfläschchen und 420 mg/Mehrfachdosisfläschchen), Endverbraucher (Fachkliniken, Krankenhäuser, onkologische Zentren und andere), Vertriebskanal (Direktausschreibung, Krankenhausapotheke, Einzelhandelsapotheke, Online-Apotheke und andere) |
|
Abgedeckte Länder |
USA, Kanada, Mexiko, Brasilien, Argentinien, Peru, restliches Südamerika, Deutschland, Frankreich, Großbritannien, Niederlande, Schweiz, Belgien, Russland, Italien, Spanien, Türkei, Ungarn, Litauen, Österreich, Irland, Norwegen, Polen, restliches Europa, China, Japan, Indien, Südkorea, Singapur, Malaysia, Australien, Thailand, Indonesien, Philippinen, Vietnam, restlicher asiatisch-pazifischer Raum, Saudi-Arabien, Vereinigte Arabische Emirate, Ägypten, Israel, Kuwait, Südafrika, restlicher Naher Osten und Afrika. |
|
Abgedeckte Marktteilnehmer |
Amgen Inc. (USA), AryoGen Pharmed (Iran), Biocon (Indien), Accord Healthcare Ltd (Großbritannien), Celltrion Healthcare Co.,Ltd. (Südkorea), Pfizer Inc. (USA), Merck Sharp & Dohme Corp., eine Tochtergesellschaft von Merck & Co., Inc. (USA), Shanghai Henlius Biotech, Inc. (China), Gedeon Richter Plc. (Ungarn), Genor Biopharma Company Ltd (China), MABION SA (Polen), Mylan NV (Großbritannien), F. Hoffmann-La Roche Ltd (Schweiz), Samsung Bioepis (Südkorea), ACROBiosystems (USA), Genentech, USA Inc. (USA), AstraZeneca (Großbritannien), Halozyme, Inc. (USA) und Teva Pharmaceuticals USA, Inc. (USA) unter anderem |
|
Marktchancen |
|
Marktdefinition
Herceptin (Trastuzumab) ist ein monoklonaler Antikörper, der durch Bindung an den humanen epidermalen Wachstumsfaktor-Rezeptor 2 (HER2) wirksame Ergebnisse erzielt. Bei mehreren Krebsarten wird das HER2-Gen überexprimiert, was aufgrund des unkontrollierten Zellwachstums zur Krebsentstehung führt. Herceptin wurde 1998 erstmals zur Behandlung von metastasiertem Brustkrebs zugelassen. Mittlerweile sind etwa fünf Herceptin-Biosimilars auf dem US-Markt zugelassen. Das Herceptin-Biosimilar ist für 70.000 USD für eine einjährige Behandlung erhältlich und hat sich als Standardbehandlung von Brustkrebs bewährt.
Globale Marktdynamik für Herceptin-Biosimilars
Treiber
- Steigende Brustkrebsrate
Der Markt für Herceptin-Biosimilars dürfte im Prognosezeitraum aufgrund einer Reihe von Schlüsselfaktoren erheblich wachsen, darunter die steigende Prävalenz von Krebserkrankungen, insbesondere Brustkrebs. Herceptin-Biosimilars können dazu beitragen, die steigende Nachfrage nach Behandlungen für HER-2-positiven Brustkrebs zu decken.
- Verbesserter Zugang zu Therapien und Kosteneffizienz
Die Verfügbarkeit von Herceptin-Biosimilars kann den Zugang zur Therapie für Patienten mit HER2-positivem Brustkrebs verbessern, da sie die finanzielle Belastung der Behandlung verringern kann. Außerdem sind Herceptin-Biosimilars im Allgemeinen günstiger als das Originalpräparat, was zu erheblichen Kosteneinsparungen für Gesundheitssysteme, Patienten und Kostenträger führen kann. Diese Faktoren sind für den Markt im Prognosezeitraum verantwortlich.
Gelegenheiten
- Schwellenmärkte schaffen Chancen
Schwellenmärkte wie der Asien-Pazifik-Raum und Lateinamerika bieten aufgrund der steigenden Zahl an Brustkrebserkrankungen und der zunehmenden Nachfrage nach kostengünstigen Behandlungsmöglichkeiten erhebliche Wachstumschancen für den Markt für Herceptin-Biosimilars.
- Ablaufdatum des Patienten
Der Patentablauf anderer Biologika im Markt für HER2-positiven Brustkrebs, wie etwa Perjeta und Kadcyla, kann für Herceptin-Biosimilars die Möglichkeit schaffen, erhebliche Marktanteile zu gewinnen.
Einschränkungen/Herausforderungen
- Begrenztes Bewusstsein
Das geringe Bewusstsein für die Verwendung von Herceptin und die strengen Vorschriften für die Zulassung und Herstellung von Herceptin-Biosimilars sind Faktoren, die das Marktwachstum behindern und die Herceptin-Biosimilars weiter herausfordern werden. Auch das Fehlen günstiger Erstattungsszenarien und der Technologiedurchdringung in den Entwicklungsländern, Sicherheitsbedenken im Zusammenhang mit Herceptin-Biosimilars und das Fehlen einer geeigneten Infrastruktur in Ländern mit niedrigem und mittlerem Einkommen werden den Markt im Prognosezeitraum 2023–2030 voraussichtlich vor Herausforderungen stellen.
Dieser Marktbericht zu Herceptin-Biosimilars enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-Export-Analysen, Produktionsanalysen, Wertschöpfungskettenoptimierungen, Marktanteilen, dem Einfluss inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neu entstehende Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um weitere Informationen zum Markt für Herceptin-Biosimilars zu erhalten, wenden Sie sich an Data Bridge Market Research, um einen Analystenbericht zu erhalten. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Jüngste Entwicklungen
- Im Juli 2021 wurde Zercepac (Trastuzumab-dkst) von Teva Pharmaceuticals von der US-amerikanischen FDA zur Behandlung von HER2-positivem Brustkrebs und HER2-positivem metastasiertem Magen- oder gastroösophagealen Übergangsadenokarzinom zugelassen.
- Im Januar 2021 gab Samsung Bioepis bekannt, dass Ontruzant (SB3) von der Europäischen Kommission zur Behandlung von HER2-positivem Brustkrebs zugelassen wurde.
- Im Januar 2020 gab Celltrion Healthcare bekannt, dass die Europäische Kommission ihr Medikament Herzuma (CT-96) zur Behandlung von HER2-positivem Brustkrebs zugelassen hat.
- Im Juni 2019 wurde Kanjinti (Trastuzumab-anns) von Amgen und Allergan von der US-amerikanischen FDA zur Behandlung von HER2-positivem Brustkrebs und HER2-positivem metastasiertem Magen- oder gastroösophagealen Übergangsadenokarzinom zugelassen.
Globaler Marktumfang für Herceptin-Biosimilars
Der Markt für Herceptin-Biosimilars ist nach Anwendung, Dosierung, Endverbrauchern und Vertriebskanälen segmentiert. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, die ihnen bei der strategischen Entscheidungsfindung zur Identifizierung der Kernanwendungen helfen.
Anwendung
- Brustkrebs
- Dickdarmkrebs
- Leukämie
- Lymphom
- Sonstiges
Dosierung
- 150 mg/Einzeldosis-Durchstechflasche
- 420 mg/Mehrfachdosis-Durchstechflasche
Endbenutzer
- Spezialkliniken
- Krankenhäuser
- Onkologische Zentren
- Sonstiges
Vertriebskanal
- Direkte Ausschreibung
- Krankenhausapotheke
- Einzelhandelsapotheke
- Online-Apotheke
- Sonstiges
Globaler Herceptin-Biosimilars-Markt – Regionale Analyse/Einblicke
Der Markt wird analysiert und es werden Einblicke in die Marktgröße und Trends nach Land, Anwendung, Dosierung, Endbenutzern und Vertriebskanal wie oben angegeben bereitgestellt.
Die im Marktbericht abgedeckten Länder sind die USA, Kanada, Mexiko, Brasilien, Argentinien, Peru, der Rest von Südamerika, Deutschland, Frankreich, Großbritannien, Niederlande, Schweiz, Belgien, Russland, Italien, Spanien, Türkei, Ungarn, Litauen, Österreich, Irland, Norwegen, Polen, der Rest von Europa, China, Japan, Indien, Südkorea, Singapur, Malaysia, Australien, Thailand, Indonesien, Philippinen, Vietnam, der Rest des asiatisch-pazifischen Raums, Saudi-Arabien, Vereinigte Arabische Emirate, Ägypten, Israel, Kuwait, Südafrika, der Rest des Nahen Ostens und Afrikas.
Nordamerika dominiert den Markt aufgrund seiner starken Basis an Gesundheitseinrichtungen, der starken Präsenz wichtiger Akteure, der außergewöhnlichen Gesundheitsinfrastruktur und der großen Zahl an Menschen mit Brustkrebs, Leukämie, Lymphomen und anderen Erkrankungen.
Im asiatisch-pazifischen Raum wird im Prognosezeitraum von 2023 bis 2030 voraussichtlich ein deutliches Wachstum erwartet. Grund dafür sind die zunehmenden Regierungsinitiativen zur Förderung der Gesundheitsversorgung, das steigende Gesundheitsbewusstsein der Bevölkerung, die wachsende Nachfrage nach fortschrittlicher Medizintechnik zur Krebsbehandlung , die große Bevölkerung und die steigende Nachfrage nach qualitativ hochwertiger Gesundheitsversorgung in der Region.
Der Länderabschnitt des Berichts enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Regulierung auf dem Inlandsmarkt, die sich auf die aktuellen und zukünftigen Trends des Marktes auswirken. Datenpunkte wie Downstream- und Upstream-Wertschöpfungskettenanalysen, technische Trends und Porters Fünf-Kräfte-Analyse sowie Fallstudien sind einige der Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Bei der Bereitstellung von Prognoseanalysen der Länderdaten werden auch die Präsenz und Verfügbarkeit globaler Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken, die Auswirkungen inländischer Zölle und Handelsrouten berücksichtigt.
Wachstum der installierten Basis der Gesundheitsinfrastruktur und Durchdringung mit neuen Technologien
Der Markt für Herceptin-Biosimilars bietet Ihnen außerdem eine detaillierte Marktanalyse zum Wachstum der Gesundheitsausgaben für Investitionsgüter in jedem Land, zur installierten Basis verschiedener Arten von Produkten für den Markt für Herceptin-Biosimilars, zum Einfluss der Technologie anhand von Lebenskurven und zu Änderungen der regulatorischen Szenarien im Gesundheitswesen und zu ihren Auswirkungen auf den Markt für Herceptin-Biosimilars.
Wettbewerbsumfeld und Herceptin-Biosimilars Marktanteilsanalyse
Die Wettbewerbslandschaft des Marktes für Herceptin-Biosimilars liefert Details zu den Wettbewerbern. Die enthaltenen Details sind Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang, Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen in Bezug auf den Markt für Herceptin-Biosimilars.
Einige der wichtigsten Akteure auf dem Markt sind:
- Amgen Inc. (USA)
- AryoGen Pharmed (Iran)
- Accord Healthcare Ltd (Großbritannien)
- Biocon (Indien)
- Celltrion Healthcare Co. Ltd. (Südkorea)
- Pfizer Inc. (USA)
- Merck Sharp & Dohme Corp., eine Tochtergesellschaft von Merck & Co., Inc. (USA)
- Shanghai Henlius Biotech, Inc. (China)
- Gedeon Richter Plc. (Ungarn)
- Genor Biopharma Company Ltd (China)
- MABION SA (Polen)
- Mylan NV (Großbritannien)
- F. Hoffmann-La Roche Ltd (Schweiz)
- Samsung Bioepis (Südkorea)
- ACROBiosystems (USA)
- Genentech, USA Inc. (USA)
- AstraZeneca (Großbritannien)
- Halozyme, Inc. (USA)
- Teva Pharmaceuticals USA, Inc. (USA)
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.