Global Fetal Monitoring Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
4.00 Billion
USD
6.57 Billion
2024
2032
USD
4.00 Billion
USD
6.57 Billion
2024
2032
| 2025 –2032 | |
| USD 4.00 Billion | |
| USD 6.57 Billion | |
|
|
|
|
Global Fetal Monitoring Market Segmentation, By Product Type (Instruments and Consumables, Ultrasound, Electronic Maternal/Fetal Monitor, Fetal Electrodes, Fetal Doppler, Telemetry Solutions, Accessories and Consumables, and Software), Portability (Portable and Non-portable), Methods (Non-Invasive and Invasive), Application (Intrapartum Fetal Monitoring and Antepartum Fetal Monitoring), End User (Hospitals, Gynaecological/Obstetrics Clinics, Homecare, and Others), Distribution Channel (Direct Tender and Retail) - Industry Trends and Forecast to 2032
Fetal Monitoring Market Size
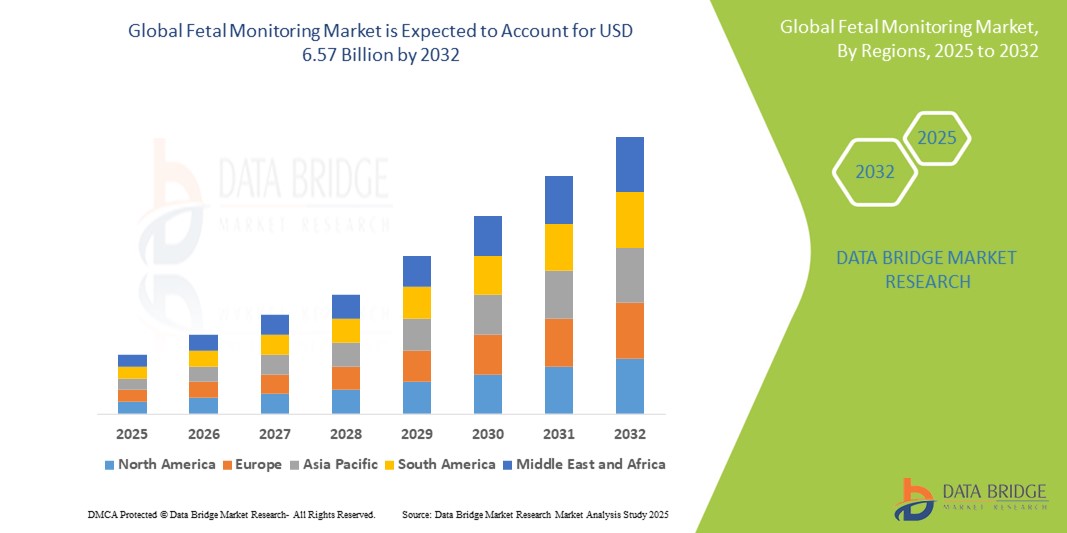
- The global Fetal Monitoring market size was valued atUSD 4.00 billion in 2024and is expected to reachUSD 6.57 billion by 2032, at aCAGR of 6.40%during the forecast period
- This growth is driven by factors such as the rising incidence of pregnancy complications, government initiatives and awareness campaigns, growing prevalence of high-risk pregnancies
Fetal Monitoring Market Analysis
- Fetal monitoring refers to monitoring the fetal heart rate and other parameters during pregnancy, labor, and delivery. It is used to assess the fetus's well-being and detect any signs of distress or complications
- Fetal monitoring can be done using electronic devices that measure the fetal heart rate and uterine contractions, or it can be done through more traditional methods such as auscultation with a stethoscope
- North America is expected to dominate the fetal monitoring market with 28.36% due to growing technological advancements and an increasing emphasis on maternal health
- Asia-Pacific is expected to be the fastest growing region in the fetal monitoring market during the forecast period due to growing integration of advanced technologies
- Antepartum Fetal Monitoring segment is expected to dominate the market with a market share of 52.8% due to its rising prevalence of chronic conditions among pregnant women, such as high blood pressure, diabetes, and obesity, has necessitated the need for enhanced monitoring solutions
Report Scope and Fetal Monitoring Market Segmentation
|
Attributes |
Fetal Monitoring Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Fetal Monitoring Market Trends
“Rise of Non-Invasive and Remote Monitoring”
- The fetal monitoring market is experiencing a shift towards non-invasive and remote monitoring solutions
- Innovations such as wireless fetal heart rate monitors and wearable devices enable continuous monitoring without the need for invasive procedures
- These advancements align with the growing demand for patient-centric care, allowing expectant mothers to monitor fetal health from the comfort of their homes, thereby enhancing convenience and reducing hospital visits
- The integration of fetal monitoring devices with telemedicine platforms facilitates real-time data transmission to healthcare providers, enabling timely interventions and personalized care plans
Fetal Monitoring Market Dynamics
Driver
“Increasing Preterm Birth Rates”
- The World Health Organization reports that approximately 13.4 million babies are born prematurely each year, accounting for about 1 in 10 live births globally
- Premature infants often require intensive care and continuous monitoring to manage potential complications, driving the demand for advanced fetal monitoring equipment
- Neonatal Intensive Care Units (NICUs) necessitate specialized monitoring systems to ensure the well-being of preterm infants, further propelling market growth
- The increasing incidence of preterm births underscores the critical need for reliable and efficient fetal monitoring solutions, acting as a significant driver for market expansion
Opportunity
“Expansion in Emerging Markets”
- Emerging economies are investing in healthcare infrastructure, leading to improved access to maternal and fetal care services
- There is a growing demand for cost-effective fetal monitoring solutions that do not compromise on quality, presenting opportunities for manufacturers to innovate and cater to these markets
- Governments and non-governmental organizations are implementing programs to enhance maternal and child health, creating a conducive environment for the adoption of fetal monitoring technologies
- According to Research and Markets, the global fetal monitoring market is expected to witness substantial growth, driven by various factors, including technological advancements and government initiatives to improve maternal and fetal care
Restraint/Challenge
“High Costs and Limited Reimbursement”
- Advanced fetal monitoring devices involve significant initial investment and maintenance expenses, which can be prohibitive for healthcare facilities, especially in low-resource settings
- Inconsistent reimbursement policies across regions create financial barriers for healthcare providers, limiting the widespread adoption of these technologies
- The availability of refurbished devices at lower prices intensifies competition, pressuring manufacturers to justify the premium pricing of new devices
- Economic instability and reduced funding for healthcare initiatives can delay the procurement of advanced monitoring systems, hindering market growth
- These financial constraints pose significant challenges to the adoption of fetal monitoring technologies, particularly in developing regions where cost is a primary concern
Fetal Monitoring Market Scope
The market is segmented on the basis of product type, portability, methods, application, end user, and distribution channel
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By Portability |
|
|
By Methods |
|
|
By Application |
|
|
By End User |
|
|
By Distribution Channel |
|
In 2025, the antepartum fetal monitoring is projected to dominate the market with a largest share in application segment
The antepartum fetal monitoring segment is expected to dominate the Fetal Monitoring market with the largest share of 52.8% in 2025 due to its rising prevalence of chronic conditions among pregnant women, such as high blood pressure, diabetes, and obesity, has necessitated the need for enhanced monitoring solutions. These conditions elevate the risk of complications during pregnancy.
The electronic maternal/fetal monitor is expected to account for the largest share during the forecast period in product type market
In 2025, the electronic maternal/fetal monitor segment is expected to dominate the market with the largest market share of 30.3% due to its growing awareness about the importance of monitoring fetal health, along with an increase in preterm births and fetal issues, is boosting the demand for fetal monitors. Moreover, technological advancements that improve these devices' accuracy, portability, and user-friendliness are making them more widely available for use in diverse healthcare settings, including at home.
Fetal Monitoring Market Regional Analysis
“North America Holds the Largest Share in the Fetal Monitoring Market”
- North America held the largest market with 28.36% of the global fetal monitoring market revenue. This dominance is attributed to advanced healthcare infrastructure and high adoption rates of medical technologies
- The region exhibits a significant number of preterm births, increasing the demand for fetal monitoring devices to ensure the health of neonates
- North America benefits from the availability of advanced fetal monitoring products, including wireless and portable devices, enhancing patient comfort and accessibility
- High healthcare spending in countries such as the U.S. and Canada supports the procurement of state-of-the-art medical equipment, including fetal monitoring systems
- Favorable regulatory frameworks and reimbursement policies in North America facilitate the widespread adoption of fetal monitoring technologies
“Asia-Pacific is Projected to Register the Highest CAGR in the Fetal Monitoring Market”
- The Asia Pacific region is expected to witness the highest compound annual growth rate driven by increasing healthcare investments and infrastructure development
- Growing awareness about maternal and fetal health is leading to increased demand for fetal monitoring devices in countries such as India, China, and Japan
- Governments in the Asia Pacific region are implementing policies to improve maternal and child health, further boosting the adoption of fetal monitoring technologies
- Rapid economic development in the region is leading to higher disposable incomes, enabling more families to access advanced healthcare services, including fetal monitoring
- The increasing prevalence of pregnancy-related complications and the expansion of healthcare facilities are contributing to the accelerated growth of the fetal monitoring market in Asia Pacific
Fetal Monitoring Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Johnson & Johnson Private Limited(U.S.)
- Abbott(U.S.)
- Medtronic(Ireland)
- Boston Scientific Corporation(U.S.)
- B. Braun Melsungen AG(Germany)
- Baxter (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- General Electric (U.S.)
- FUJIFILM Holdings Corporation (Japan)
- Siemens Healthcare GmbH (Germany)
- Analogic Corporation (U.S.)
- Getinge AB (Sweden)
- Natus Medical Incorporated. (Sweden)
- OSI Systems, Inc. (U.S.)
- Natus Medical Incorporated (U.S.)
- EDAN Instruments, Inc. (China)
- The Cooper Companies Inc. (U.S.)
- MEDGYN PRODUCTS, INC. (U.S.)
- Dixion (Germany)
- Advanced Instruments (U.S.)
- Shenzhen Bestman Instrument Co.,Ltd., (China)
Latest Developments in Global Fetal Monitoring Market
- In April 2024, GE Healthcare introduced the AI-driven Voluson Signature 20 and 18 ultrasound systems to enhance imaging in women’s health. This launch benefits the fetal monitoring equipment sector by improving the accuracy and efficiency of ultrasound imaging through sophisticated artificial intelligence
- In February 2024, GE Healthcare received FDA 510(k) clearance for its Novii+ Wireless Patch Solution. This advanced monitors noninvasively tracks fetal heart rate, maternal heart rate, and uterine activity. The belt-free, wireless design enhances maternal mobility, potentially improving labor comfort and reducing labor duration
- In February 2024, GE Healthcare announced that its Novii+ Wireless Maternal and Fetal Monitoring Equipment System received FDA clearance to monitor nearly 95% of all eligible births in the U.S
- In November 2022, The Cooper Companies Inc. acquired Cook Medical’s Reproductive Health division, which specializes in producing minimally invasive medical devices for the fertility, obstetrics, and gynecology sectors
- In February 2021, CooperSurgical initiated a multi-year strategic partnership with Virtus Health, a leading global provider of assisted reproductive services. This collaboration, which begins this month, aims to foster innovation, enhance digitalization, and improve fertility treatment advancements.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.