Global External Ventricular Drain Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
2.92 Billion
USD
4.38 Billion
2024
2032
USD
2.92 Billion
USD
4.38 Billion
2024
2032
| 2025 –2032 | |
| USD 2.92 Billion | |
| USD 4.38 Billion | |
|
|
|
|
Globale Marktsegmentierung für externe Ventrikeldrainagen nach Produkttyp (Ventrikeldrainage-Set und Zubehör), Indikationsanalyse (Tumor der hinteren Schädelgrube, Hämatome der hinteren Schädelgrube, Obstruktion des IV. Ventrikels, Schädelverletzungen, subdurale Hämatome, Meningitis, Subarachnoidalblutung in die Zerebrospinalflüssigkeit und andere), Anwendung (Hydrozephalus, Reye-Syndrom, Subarachnoidalblutung, Schädel-Hirn-Trauma und andere), Endbenutzer (Krankenhäuser, Fachkrankenhäuser, Fachkliniken und andere) – Branchentrends und Prognose bis 2032
Externe Ventrikeldrainage Marktgröße
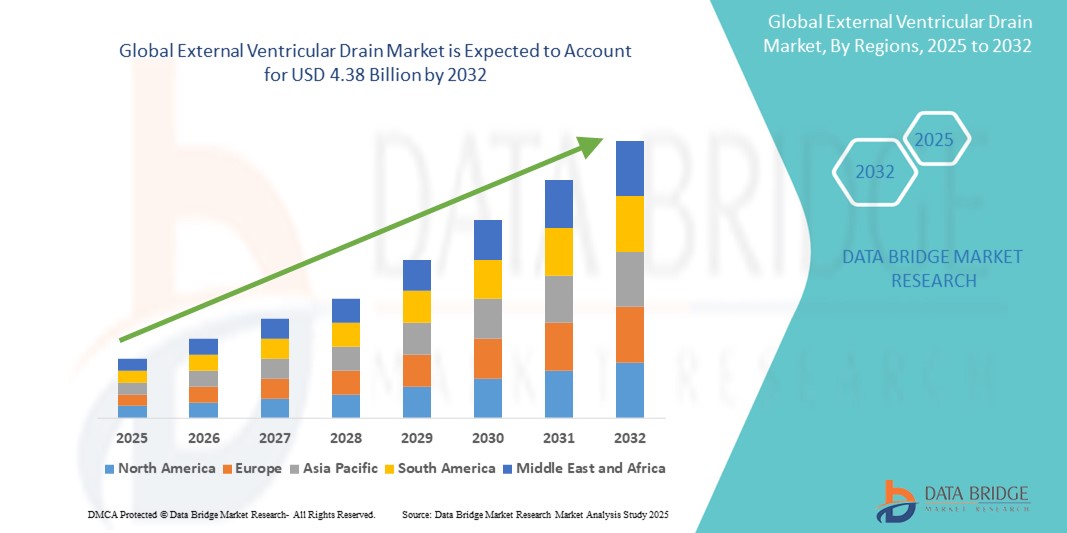
- Der globale Markt für externe Ventrikeldrainage wird im Jahr 2024 auf 2,92 Milliarden US-Dollar geschätzt und soll bis 2032 einen Wert von 4,38 Milliarden US-Dollar erreichen , was einer jährlichen Wachstumsrate (CAGR) von 5,2 % im Prognosezeitraum entspricht.
- Das Marktwachstum ist größtenteils auf die zunehmende Häufigkeit traumatischer Hirnverletzungen, intrakranieller Blutungen und Hydrozephalus zurückzuführen, die in der Intensivpflege eine sofortige Behandlung der Zerebrospinalflüssigkeit über externe Drainagesysteme erfordern.
- Darüber hinaus verbessern das steigende Bewusstsein für die neurokritische Versorgung sowie Fortschritte im Design von EVD-Geräten wie antimikrobiellen Kathetern und integrierter Drucküberwachung die Behandlungsergebnisse. Diese medizinischen und technologischen Fortschritte beschleunigen die Einführung externer Ventrikeldrainagen und fördern damit das Wachstum der Branche erheblich.
Externe Ventrikeldrainage Marktanalyse
- Externe Ventrikeldrainagen (EVDs), die zur Linderung von erhöhtem intrakraniellen Druck und zur Ableitung von Liquor cerebrospinalis (CSF) eingesetzt werden, sind in der Neurochirurgie und Intensivmedizin aufgrund ihrer unmittelbaren Wirksamkeit und Präzision bei der Überwachung der intrakraniellen Dynamik bei der Behandlung von Erkrankungen wie Schädel-Hirn-Trauma, Hydrozephalus und Subarachnoidalblutungen zunehmend unverzichtbar.
- Die steigende Nachfrage nach EVD-Systemen wird vor allem durch die weltweit steigende Zahl neurokritischer Erkrankungen, das wachsende Bewusstsein für ein frühzeitiges Management des intrakraniellen Drucks und technologische Fortschritte, die die Sicherheit und Präzision von Drainage- und Überwachungssystemen verbessern, angetrieben.
- Nordamerika dominierte den Markt für externe Ventrikeldrainage mit dem größten Umsatzanteil von 39,2 % im Jahr 2024. Dieser Markt zeichnet sich durch eine fortschrittliche Gesundheitsinfrastruktur, höhere Raten neurochirurgischer Eingriffe und die Präsenz wichtiger Hersteller medizinischer Geräte aus. Die USA sind aufgrund der besseren Erreichbarkeit von Intensivstationen und der frühen Einführung verbesserter Drainagetechnologien bei der Einführung von EVD führend.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum die am schnellsten wachsende Region im Markt für externe Ventrikeldrainage sein, da sich die Gesundheitsinfrastruktur verbessert, die Gesundheitsausgaben steigen und die Zahl der Hirnverletzungen in den sich schnell urbanisierenden Regionen zunimmt.
- Das Segment der Ventrikeldrainage-Sets dominierte den Markt für externe Ventrikeldrainagen mit einem Marktanteil von 69,9 % im Jahr 2024, was auf die weit verbreitete Verwendung bei neurochirurgischen Notfalleingriffen und das standardisierte Design zurückzuführen ist, das eine effiziente und sterile Liquordrainage gewährleistet.
Berichtsumfang und Marktsegmentierung für externe Ventrikeldrainage
|
Eigenschaften |
Externe Ventrikeldrainage – Wichtige Markteinblicke |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für externe Ventrikeldrainage
„Fortschritte bei EVD-Systemen für Präzision und Infektionskontrolle“
- Ein bedeutender und zunehmender Trend auf dem globalen Markt für externe Ventrikeldrainage (EVD) ist die technologische Weiterentwicklung von EVD-Systemen zur Verbesserung von Präzision, Patientensicherheit und Infektionskontrolle in der neurologischen Intensivpflege. Innovationen wie antimikrobiell beschichtete Katheter, digitale Drainageüberwachung und geschlossene Kreislaufsysteme gewinnen bei Gesundheitsdienstleistern an Bedeutung, da sie Komplikationen reduzieren und das Liquormanagement optimieren können.
- Medtronic und Integra LifeSciences bieten beispielsweise EVD-Systeme mit antimikrobiellen Eigenschaften und integrierten Drucksensoren an, die eine Echtzeitüberwachung des intrakraniellen Drucks (ICP) ermöglichen und so das Risiko katheterassoziierter Infektionen minimieren. Ebenso bietet die Spiegelberg GmbH fortschrittliche Drainageeinheiten mit einstellbaren Druckventilen und visuellen Anzeigen an, die eine präzise Liquorableitung gewährleisten.
- Diese intelligenten Systeme steigern nicht nur die klinische Effizienz, sondern tragen auch zur Patientensicherheit bei, indem sie Frühwarnfunktionen bieten und das Risiko einer Über- oder Unterdrainage reduzieren. Digitale Systeme ermöglichen zudem eine kontinuierliche Drucküberwachung und automatisieren den Liquorabfluss, wodurch manuelle Fehler minimiert werden.
- Die Integration von EVD-Systemen in umfassendere Neuromonitoring-Plattformen und elektronische Gesundheitsakten (EHRs) verbessert zudem die Datendokumentation und die interdisziplinäre Entscheidungsfindung und trägt so zu besseren Patientenergebnissen bei.
- Dieser Trend zu präziseren, automatisierten und infektionsresistenten EVD-Lösungen verändert die Art und Weise, wie neurochirurgische Teams das intrakraniale Druckmanagement angehen. Hersteller wie B. Braun und Sophysa investieren daher in EVD-Geräte der nächsten Generation, die auf intuitive Steuerung, verbesserte Sicherheit und Kompatibilität mit Intensivstationen setzen.
- Die Nachfrage nach solchen fortschrittlichen EVD-Technologien wächst in Krankenhäusern der tertiären Versorgung und Traumazentren rasant, da Kliniker bei neurokritischen Eingriffen zunehmend Wert auf Infektionskontrolle, Echtzeitüberwachung und standardisierte Behandlungsprotokolle legen.
Marktdynamik für externe Ventrikeldrainage
Treiber
„Zunahme neurokritischer Störungen und chirurgischer Eingriffe“
- Die zunehmende Zahl neurologischer Notfälle wie Schädel-Hirn-Traumata, Hydrozephalus und Subarachnoidalblutungen ist ein Haupttreiber des Wachstums im Markt für externe Ventrikeldrainage. EVD-Systeme gelten als unverzichtbare Instrumente zur Behandlung von akutem Hirndruck und zur Liquordrainage in der neurologischen Intensivmedizin.
- Globale Traumadaten deuten beispielsweise auf einen Anstieg der Krankenhauseinweisungen aufgrund von Kopfverletzungen hin, insbesondere aufgrund von Verkehrsunfällen und Stürzen. In vielen Fällen ist die sofortige Platzierung eines EVD erforderlich, um Hirnschäden zu verhindern. Mit dem weltweiten Ausbau der neurologischen Versorgung steigt auch die Nachfrage nach effektiven EVD-Systemen.
- Darüber hinaus verbessert die zunehmende Verfügbarkeit neurochirurgischer Expertise und moderner Intensivpflegeinfrastruktur, insbesondere in entwickelten Ländern, den Zugang zu zeitnahen und sicheren EVD-Verfahren. Der zunehmende Einsatz von EVDs in der postoperativen neurochirurgischen Versorgung und zur Überwachung von Patienten mit Hirntumoren oder Blutungen unterstreicht ihre klinische Bedeutung zusätzlich.
- Da das Bewusstsein dafür zunimmt und die protokollbasierte Versorgung auf Intensivstationen zum Standard wird, wird die Rolle von EVD-Systemen immer stärker in die Arbeitsabläufe der Notfall- und chirurgischen Versorgung integriert, was zu einer anhaltenden Marktakzeptanz führt.
Einschränkung/Herausforderung
„Infektionsrisiko und Fachkräftebedarf“
- Trotz ihres kritischen klinischen Nutzens bergen externe Ventrikeldrainagen ein hohes Risiko für Komplikationen, insbesondere für katheterassoziierte Infektionen (CAIs) wie Ventrikulitis und Meningitis, die weiterhin erhebliche Hindernisse für ihre breite Anwendung darstellen.
- Beispielsweise können unsachgemäße Handhabung, längerer Kathetergebrauch oder die mangelnde Einhaltung von Infektionskontrollprotokollen die Infektionsrate erhöhen, was zu längeren Aufenthalten auf der Intensivstation und höheren Behandlungskosten führt. Diese Risiken sind besonders in Gesundheitseinrichtungen mit begrenzten Ressourcen oder unzureichend geschultem Personal besorgniserregend.
- Darüber hinaus erfordert die sichere Platzierung und Wartung von EVDs eine spezielle neurochirurgische Ausbildung und eine kontinuierliche Überwachung durch erfahrenes Intensivpersonal, was die Einführung in kleineren Krankenhäusern oder ländlichen Gebieten mit einem Mangel an qualifiziertem Personal einschränken kann.
- Regulierungs- und Erstattungsprobleme in Schwellenländern sowie die relativ hohen Kosten moderner EVD-Systeme erschweren die Marktdurchdringung zusätzlich.
- Die Bewältigung dieser Herausforderungen durch die Entwicklung benutzerfreundlicher, infektionsresistenter Geräte, verbesserter klinischer Schulungsprogramme und erschwinglicher Produktinnovationen wird von entscheidender Bedeutung sein, um einen breiteren Zugang zu ermöglichen und das globale Marktwachstum voranzutreiben.
Externer Ventrikeldrain Marktumfang
Der Markt ist nach Produkttyp, Indikationsanalyse, Anwendung und Endbenutzer segmentiert.
- Nach Produkttyp
Der Markt für externe Ventrikeldrainagen ist nach Produkttyp in Ventrikeldrainage-Sets und Zubehör unterteilt. Das Segment der Ventrikeldrainage-Sets dominierte den Markt mit dem größten Umsatzanteil von 69,9 % im Jahr 2024, was auf seine wichtige Rolle bei der Behandlung von erhöhtem intrakraniellen Druck (ICP) und der Drainage von Liquor cerebrospinalis (CSF) in der neurologischen Intensivmedizin zurückzuführen ist. Diese Sets werden aufgrund ihrer gebrauchsfertigen Beschaffenheit, sterilen Verpackung und Kompatibilität mit den meisten neurochirurgischen Eingriffen bevorzugt.
Im Zubehörsegment wird im Prognosezeitraum voraussichtlich ein stetiges Wachstum erwartet, das auf die zunehmende Einführung anpassbarer Drainagesysteme zurückzuführen ist, die moderne Druckwandler, antimikrobielle Komponenten und zusätzliche Schlauchoptionen für eine flexible klinische Anwendung und verbesserte Infektionskontrolle umfassen.
- Nach Indikationsanalyse
Basierend auf der Indikationsanalyse ist der Markt für externe Ventrikeldrainagen in die Bereiche Tumor der hinteren Schädelgrube, Hämatome der hinteren Schädelgrube, Verschluss des IV-Ventrikels, Schädelverletzungen, subdurale Hämatome, Meningitis, Subarachnoidalblutung in die Zerebrospinalflüssigkeit und weitere unterteilt. Das Segment der Subarachnoidalblutung in die Zerebrospinalflüssigkeit dominierte den Markt mit dem größten Anteil von 34,6 % im Jahr 2024, was auf die hohe Prävalenz aneurysmatischer Subarachnoidalblutungen und die dringende Notwendigkeit einer schnellen intrakraniellen Druckregulierung bei betroffenen Patienten zurückzuführen ist. Das schnelle Auftreten von Komplikationen in diesen Fällen macht die Platzierung einer externen Ventrikeldrainage zu einem entscheidenden Bestandteil der Akutintervention.
Das Segment der Schädelverletzungen dürfte im Prognosezeitraum das schnellste Wachstum verzeichnen. Grund dafür sind die weltweit steigenden Fälle traumatischer Hirnverletzungen, insbesondere in Regionen mit niedrigem und mittlerem Einkommen, wo die Zahl der Autounfälle und Stürze zunimmt.
- Nach Anwendung
Der Markt für externe Ventrikeldrainage ist nach Anwendung in Hydrozephalus, Reye-Syndrom, Subarachnoidalblutung, Schädel-Hirn-Trauma und weitere Bereiche unterteilt. Das Segment Schädel-Hirn-Trauma hatte 2024 mit 39,2 % den größten Marktanteil, bedingt durch die weltweit steigende Zahl von Schädel-Hirn-Traumata aufgrund von Unfällen, Sportverletzungen und Kampftraumata. EVD-Systeme werden häufig eingesetzt, um den ICP zu kontrollieren und sekundäre Hirnverletzungen in diesen Fällen der Intensivpflege zu verhindern.
Das Hydrozephalus-Segment dürfte im Prognosezeitraum mit einer erheblichen durchschnittlichen jährlichen Wachstumsrate wachsen. Dies wird durch die wachsende Zahl von Kindern und Geriatern unterstützt, die unter einer Ansammlung von Zerebrospinalflüssigkeit leiden, sowie durch das steigende Bewusstsein für rechtzeitige chirurgische Eingriffe mittels EVD.
- Nach Endbenutzer
Der Markt für externe Ventrikeldrainage ist nach Endnutzern in Krankenhäuser, Fachkrankenhäuser, Fachkliniken und andere Bereiche unterteilt. Das Krankenhaussegment dominierte den Markt mit dem größten Umsatzanteil von 61,7 % im Jahr 2024, was auf die Präsenz neurologischer Intensivstationen, geschultes medizinisches Personal und die Verfügbarkeit einer umfassenden postoperativen Versorgungsinfrastruktur in Krankenhäusern mit mehreren Fachrichtungen zurückzuführen ist. Krankenhäuser sind in der Regel die erste Anlaufstelle für die Notfallversorgung bei Neurotraumata und damit die größten Abnehmer von EVD-Systemen.
Das Segment der Spezialkrankenhäuser dürfte im Prognosezeitraum stark wachsen und von der zunehmenden Konzentration auf Neurologie und Neurochirurgie als Spezialgebiete sowie den steigenden Investitionen in eine fortschrittliche Infrastruktur für die neurokritische Versorgung sowohl im öffentlichen als auch im privaten Gesundheitswesen profitieren.
Externe Ventrikeldrainage Marktregionale Analyse
- Nordamerika dominierte den Markt für externe Ventrikeldrainage mit dem größten Umsatzanteil von 39,2 % im Jahr 2024, gekennzeichnet durch eine fortschrittliche Gesundheitsinfrastruktur, höhere Raten neurochirurgischer Eingriffe und die Präsenz wichtiger Hersteller medizinischer Geräte
- Gesundheitsdienstleister in der Region legen Wert auf schnelle Intervention und Präzision bei der Behandlung des intrakraniellen Drucks, was zu einem weit verbreiteten Einsatz externer Ventrikeldrainagen in der Notaufnahme und auf der Intensivstation führt
- Diese starke Akzeptanz wird durch ein gut etabliertes Gesundheitssystem, die zunehmende Anzahl neurochirurgischer Eingriffe und die Präsenz großer Hersteller medizinischer Geräte weiter unterstützt, wodurch EVDs als Standardinstrument für die Behandlung komplexer neurokritischer Erkrankungen in Krankenhäusern und Traumazentren positioniert werden.
Markteinblick in die USA für externe Ventrikeldrainagen
Der US-Markt für externe Ventrikeldrainagen erzielte 2024 mit 80,3 % den größten Umsatzanteil in Nordamerika, was auf die fortschrittliche Gesundheitsinfrastruktur des Landes und die hohe Anzahl neurochirurgischer Eingriffe zurückzuführen ist. Die zunehmende Zahl traumatischer Hirnverletzungen, Schlaganfälle und Subarachnoidalblutungen treibt die Einführung von EVDs sowohl in der Notaufnahme als auch auf Intensivstationen voran. Die starke Präsenz wichtiger Marktteilnehmer und die frühzeitige Einführung technologisch fortschrittlicher Drainagesysteme unterstützen ein stetiges Marktwachstum, während staatliche Förderung und klinische Forschung in der neurokritischen Versorgung die Nachfrage weiter ankurbeln.
Einblicke in den europäischen Markt für externe Ventrikeldrainage
Der europäische Markt für externe Ventrikeldrainage wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen, vor allem aufgrund des steigenden Bewusstseins für die neurokritische Versorgung und standardisierter Behandlungsprotokolle bei Hirnverletzungen. Strenge klinische Sicherheitsvorschriften und kontinuierliche Investitionen in moderne neurochirurgische Geräte fördern die Einführung von EVD-Systemen. Krankenhäuser in der gesamten Region integrieren moderne EVD-Lösungen in Trauma- und neurointensivmedizinische Stationen. Sowohl in städtischen medizinischen Zentren als auch in Spezialkliniken in Mittel- und Osteuropa ist eine steigende Nachfrage zu beobachten.
Markteinblick in Großbritannien für externe Ventrikeldrainagen
Der britische Markt für externe Ventrikeldrainage wird im Prognosezeitraum voraussichtlich mit einer bemerkenswerten jährlichen Wachstumsrate wachsen, angetrieben durch die steigende Zahl neurochirurgischer Eingriffe und die staatlichen Bemühungen zur Stärkung der Intensivmedizin. Der Fokus des Landes auf die Verbesserung der Infrastruktur für Schlaganfall- und Traumaversorgung führt zu einem verstärkten Einsatz von EVD-Systemen. Darüber hinaus dürften etablierte Gesundheitseinrichtungen und expandierende neurochirurgische Abteilungen ein nachhaltiges Marktwachstum unterstützen, insbesondere in Universitätskliniken und NHS-finanzierten Krankenhäusern.
Markteinblick für externe Ventrikeldrainage in Deutschland
Der deutsche Markt für externe Ventrikeldrainagen wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen, unterstützt durch hohe Standards in der Krankenhausversorgung und den starken Fokus des Landes auf Präzisionsneurochirurgie. Die wachsende ältere Bevölkerung Deutschlands sowie die hohe Inzidenz von Schlaganfällen und Hirnblutungen tragen zu einer steigenden Nachfrage nach EVDs bei. Darüber hinaus fördern innovationsgetriebene Kooperationen zwischen Krankenhäusern und Medizintechnikunternehmen die Entwicklung und Einführung digitaler und antimikrobieller EVD-Systeme sowohl in Tertiär- als auch in Universitätskliniken.
Markteinblicke für externe Ventrikeldrainagen im asiatisch-pazifischen Raum
Der Markt für externe Ventrikeldrainage im asiatisch-pazifischen Raum dürfte im Prognosezeitraum von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 24,1 % wachsen. Dies ist auf die steigenden Raten traumatischer Hirnverletzungen, den verbesserten Zugang zur Gesundheitsversorgung und steigende Investitionen in neurokritische Versorgungseinrichtungen in Ländern wie China, Indien und Japan zurückzuführen. Staatliche Reformen der Gesundheitsinfrastruktur sowie die wachsende Präsenz globaler und nationaler Hersteller medizinischer Geräte machen EVD-Systeme zugänglicher. Die zunehmende Anzahl tertiärer Versorgungszentren und Neurochirurgen in der Region fördert die Marktdurchdringung zusätzlich.
Markteinblick in Japan für externe Ventrikeldrainage
Der japanische Markt für externe Ventrikeldrainagen gewinnt aufgrund des technologisch fortschrittlichen Gesundheitssektors des Landes und der zunehmenden Prävalenz zerebrovaskulärer Erkrankungen an Dynamik. Japans alternde Bevölkerung und der hohe Standard der neurochirurgischen Versorgung sind Schlüsselfaktoren für die Einführung externer Ventrikeldrainagen, insbesondere in städtischen Krankenhäusern und akademischen medizinischen Zentren. Die Integration digitaler Überwachungsfunktionen in Drainagesysteme und die Einhaltung strenger klinischer Sicherheitsstandards beschleunigen den Übergang zu intelligenteren und automatisierten EVD-Lösungen.
Markteinblick für externe Ventrikeldrainage in Indien
Der indische Markt für externe Ventrikeldrainage hatte 2024 den größten Marktanteil im asiatisch-pazifischen Raum. Dies ist auf die rasante Urbanisierung, die steigende Zahl von Kopfverletzungen und den verbesserten Zugang zur neurochirurgischen Versorgung in öffentlichen und privaten Krankenhäusern zurückzuführen. Indien verzeichnet eine zunehmende Verbreitung von EVD-Systemen in Traumazentren, insbesondere im Rahmen der staatlichen Bemühungen zur Verbesserung der neurologischen Notfallversorgung. Die Verfügbarkeit erschwinglicher, lokal hergestellter EVD-Kits und der Ausbau der tertiären Versorgungsinfrastruktur sind wichtige Wachstumsfaktoren im Land.
Marktanteil externer Ventrikeldrainagen
Die Branche der externen Ventrikeldrainage wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Berlin Heart (Berlin)
- Terumo Corporation (Japan)
- ABIOMED (USA)
- Johnson & Johnson Services, Inc. (USA)
- Abbott (USA)
- Getinge AB (Schweden)
- Evaheart, Inc. (USA)
- Neuromedex GmbH (Deutschland)
- Medtronic (USA)
- Sophysa (Frankreich)
- Dispomedica GmbH (Deutschland)
- Fuji Systems (Japan)
- Integra LifeSciences Corporation (USA)
- B. Braun SE (Deutschland)
- SILMAG SA (Argentinien)
- Möller Medical GmbH (Deutschland)
- Zebra Technologies Corp. (USA)
- Arkis BioSciences Inc. (USA)
- Shandong Freda Medical Device Co., Ltd. (China)
Was sind die jüngsten Entwicklungen auf dem globalen Markt für externe Ventrikeldrainage?
- Im März 2024 kündigte Medtronic plc, ein weltweit führendes Medizintechnikunternehmen, die Einführung seines antimikrobiellen externen Ventrikeldrainagesystems der nächsten Generation an, das Katheter-assoziierte Infektionen (CAIs) in der neurologischen Intensivmedizin reduzieren soll. Das fortschrittliche EVD-System verfügt über silberimprägnierte Katheter und eine verbesserte Durchflussregulierung und unterstreicht Medtronics kontinuierliches Engagement für die Verbesserung der Patientensicherheit und der Behandlungsergebnisse in der Intensivmedizin. Diese Markteinführung unterstreicht die führende Rolle des Unternehmens in der Infektionsprävention und neurochirurgischen Innovation.
- Im Februar 2024 führte die Integra LifeSciences Holdings Corporation im Rahmen eines Pilotprogramms zur Verbesserung des intrakraniellen Druckmanagements eine digitale EVD-Überwachungsplattform in ausgewählten europäischen Krankenhäusern ein. Das neue System integriert Echtzeit-Druckmessungen und automatisierte Warnmeldungen, um menschliche Fehler zu reduzieren und zeitnahe Interventionen zu ermöglichen. Das Pilotprojekt stellt einen wichtigen Schritt zur Digitalisierung neurokritischer Arbeitsabläufe dar und unterstützt Integras umfassende Strategie, intelligente, datengesteuerte Lösungen für die Neurochirurgie anzubieten.
- Im Dezember 2023 brachte die Spiegelberg GmbH & Co. KG, ein deutscher Hersteller neurochirurgischer Geräte, eine verbesserte Ventrikeldrainage-Steuereinheit mit integrierten Sicherheitsventilen und präziser Druckeinstellung auf den Markt. Dieses Upgrade ist auf Intensivstationen zugeschnitten und zielt darauf ab, Komplikationen zu minimieren und die Drainagegenauigkeit zu verbessern. Spiegelbergs Engagement für Innovation und Qualität stärkt seine Präsenz auf den EVD-Märkten in Europa und im Nahen Osten.
- Im Oktober 2023 gab Sophysa, ein auf Neurochirurgie spezialisiertes französisches Medizintechnikunternehmen, eine Zusammenarbeit mit führenden Universitätskliniken bekannt, um die Wirksamkeit seiner programmierbaren EVD-Systeme bei der Behandlung von pädiatrischem Hydrozephalus zu evaluieren. Diese Partnerschaft unterstreicht den wachsenden Fokus auf anpassbare Lösungen für komplexe neurologische Erkrankungen und positioniert Sophysa als Schlüsselakteur bei der Entwicklung spezialisierter Drainagesysteme für gefährdete Patientengruppen.
- Im August 2023 startete die B. Braun Melsungen AG eine klinische Schulungskampagne im asiatisch-pazifischen Raum, um den sicheren und standardisierten Einsatz von EVD-Systemen in Trauma- und neurochirurgischen Zentren zu unterstützen. Die Initiative umfasst praxisorientierte Schulungsmodule, digitale Ressourcen und regionale Partnerschaften mit Krankenhäusern, um klinische Ergebnisse und Infektionskontrollpraktiken zu verbessern. Diese Initiative unterstreicht die Strategie von B. Braun, Produktinnovation mit klinischer Unterstützung zu verbinden, um seine Präsenz in aufstrebenden Märkten der neurologischen Intensivmedizin auszubauen.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.