Global Endotoxin And Pyrogen Testing Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1.27 Billion
USD
2.98 Billion
2024
2032
USD
1.27 Billion
USD
2.98 Billion
2024
2032
| 2025 –2032 | |
| USD 1.27 Billion | |
| USD 2.98 Billion | |
|
|
|
|
Globale Marktsegmentierung für Endotoxin- und Pyrogentests nach Produkten und Dienstleistungen (Nachweiskits und Reagenzien, Instrumente und Systeme, Endotoxin- und Pyrogentestdienste sowie Verbrauchsmaterialien und Zubehör), Testtyp (Limulus-Amöbozytenlysat (LAL)-Test, TAL-Tests, Monozytenaktivierungstest (MAT), rekombinanter C (RFC)-Assay, In-vitro- und Kaninchen-Pyrogentest), Typ (vorgefertigte Endotoxin- und Pyrogentests, Proendotoxin- und Pyrogentests und kombinierte Endotoxin- und Pyrogentests), Produktkategorie (Clean Labeled Ingredient und konventionell), Form (Pulver und Flüssigkeit), Anwendung (Pharmazeutische Herstellung, Herstellung medizinischer Geräte, Rohstoffproduktion und Verpackungsherstellung), Methode (Gel-Clot-Endotoxin- und Pyrogentest, chromogener Endotoxin- und Pyrogentest und turbidimetrischer Endotoxin- und Pyrogentest), Kaufmodus (Große Gruppe, Mittlere und kleine Gruppe und Einzelperson), Endprodukt (Impfstoffe und/oder CGT, Biologika, Injektionsmittel und andere), Endbenutzer (Pharmaunternehmen, Biotechnologieunternehmen, biomedizinische Unternehmen, Medizingerätehersteller, Auftragsforschungsinstitute (CROs), Auftragshersteller (CMOs) und andere) – Branchentrends und Prognose bis 2032
Marktgröße für Endotoxin- und Pyrogentests
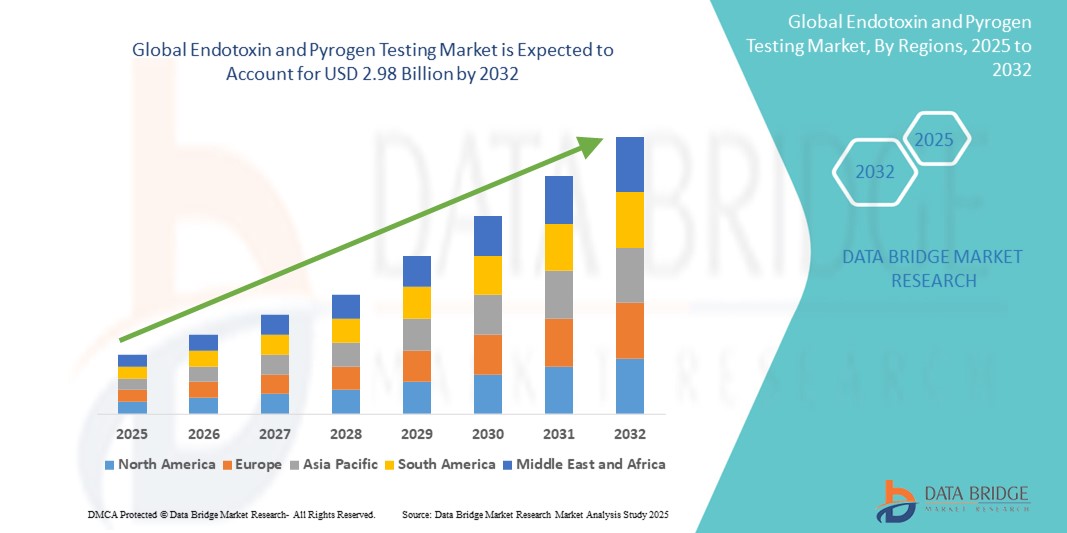
- Der globale Markt für Endotoxin- und Pyrogentests wurde im Jahr 2024 auf 1,27 Milliarden US-Dollar geschätzt und dürfte bis 2032 2,98 Milliarden US-Dollar erreichen , was einer jährlichen Wachstumsrate von 11,3 % im Prognosezeitraum entspricht.
- Das Marktwachstum wird maßgeblich durch die zunehmende Einführung und den technologischen Fortschritt in der pharmazeutischen Qualitätskontrolle und den Biosicherheitsprozessen vorangetrieben, was zu einer stärkeren Digitalisierung und Automatisierung in der Herstellung von Pharmazeutika, Biotechnologie und Medizinprodukten führt.
- Darüber hinaus etabliert die steigende Nachfrage nach präzisen, schnellen und gesetzeskonformen Endotoxin- und Pyrogen-Nachweismethoden Endotoxin- und Pyrogen-Tests als wichtigen Bestandteil moderner Produktionsabläufe für Pharmazeutika und Medizinprodukte. Diese konvergierenden Faktoren beschleunigen die Einführung von Endotoxin- und Pyrogen-Testlösungen und fördern damit das Wachstum der Branche erheblich.
Marktanalyse für Endotoxin- und Pyrogentests
- Endotoxin- und Pyrogentests bieten durch den Nachweis schädlicher bakterieller Endotoxine und Pyrogene in pharmazeutischen und medizinischen Produkten entscheidende biologische Sicherheit und sind im modernen Gesundheitswesen, in der Biopharmazie und bei der Geräteherstellung aufgrund ihrer Rolle bei der Gewährleistung der Einhaltung gesetzlicher Vorschriften und der Patientensicherheit zunehmend von entscheidender Bedeutung.
- Die steigende Nachfrage nach Endotoxin- und Pyrogentests wird vor allem durch die expandierende biopharmazeutische Industrie, die steigende Zahl chronischer Krankheiten, die injizierbare Therapien erfordern, und die verstärkte behördliche Kontrolle der Kontaminationskontrolle bei injizierbaren Medikamenten, Impfstoffen und implantierbaren Geräten angetrieben.
- Nordamerika dominierte den Markt für Endotoxin- und Pyrogentests mit dem größten Umsatzanteil von 40,01 % im Jahr 2024. Dies ist geprägt durch die frühzeitige Einführung regulatorischer Vorschriften, hohe Investitionen in die biotechnologische Forschung und die Präsenz wichtiger Akteure, die fortschrittliche Testlösungen anbieten. Die USA eroberten 81 % des regionalen Marktes, angetrieben durch einen Anstieg der Biologikaproduktion und die zunehmende Einführung automatisierter und rekombinanter Testtechnologien in GMP-zertifizierten Laboren.
- Der asiatisch-pazifische Raum wird im Prognosezeitraum voraussichtlich die am schnellsten wachsende Region im Markt für Endotoxin- und Pyrogentests sein, mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 24,02 % zwischen 2025 und 2032. Dies ist auf die schnelle Industrialisierung, den Ausbau der pharmazeutischen Produktionskapazitäten und die zunehmende staatliche Unterstützung der Qualitätskontrollinfrastruktur in Ländern wie China, Indien und Japan zurückzuführen.
- Das Segment der Nachweiskits und Reagenzien dominierte den Markt für Endotoxin- und Pyrogentests mit einem Anteil von 46,8 % im Jahr 2024 aufgrund ihrer weit verbreiteten Anwendung, Benutzerfreundlichkeit und entscheidenden Rolle in routinemäßigen Qualitätssicherungsprotokollen bei Pharma- und Biotechnologieunternehmen.
Berichtsumfang und Marktsegmentierung für Endotoxin- und Pyrogentests
|
Eigenschaften |
Wichtige Markteinblicke in Endotoxin- und Pyrogentests |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für Endotoxin- und Pyrogentests
„ Wachsende Nachfrage nach Genauigkeit, Konformität und schnellen Testlösungen “
- Ein bedeutender und sich beschleunigender Trend im asiatisch-pazifischen Markt für Endotoxin- und Pyrogentests ist die zunehmende Betonung von Genauigkeit, schnellen Durchlaufzeiten und der Einhaltung gesetzlicher Vorschriften bei der Herstellung von Arzneimitteln und Medizinprodukten. Diese Nachfrage treibt die Entwicklung und Einführung hochempfindlicher und standardisierter Testlösungen voran.
- Führende Akteure führen beispielsweise Endotoxintests auf Basis des rekombinanten Faktors C (rFC) ein, die den Einsatz tierischer Reagenzien überflüssig machen und gleichzeitig präzise und konsistente Ergebnisse gewährleisten – und dabei sowohl ethischen als auch regulatorischen Bedenken Rechnung tragen.
- Technologische Fortschritte bei automatisierten Systemen ermöglichen es Laboren zudem, Endotoxin-Nachweisprozesse zu optimieren, manuelle Eingriffe zu reduzieren und die Reproduzierbarkeit zu verbessern. Automatisierte Plattenlesegeräte und kartuschenbasierte Nachweiskits erfreuen sich aufgrund ihrer benutzerfreundlichen Bedienung und minimalen Fehlertoleranz zunehmender Beliebtheit.
- Die Integration fortschrittlicher Software in Prüfgeräte ermöglicht eine bessere Rückverfolgbarkeit, Echtzeitüberwachung und einfachere Datenberichterstattung, die für die Einhaltung der GMP-Vorschriften entscheidend sind. Dies ist insbesondere für Pharmaunternehmen relevant, die die Produktion steriler Injektionspräparate, Impfstoffe und Biologika skalieren.
- Darüber hinaus hat die zunehmende Zusammenarbeit zwischen regionalen Regierungen und Pharmaherstellern zur Verbesserung der Gesundheitsinfrastruktur – insbesondere nach COVID-19 – den Bedarf an zuverlässigen Endotoxin- und Pyrogentestprotokollen entlang der gesamten Lieferkette beschleunigt.
- Infolgedessen konzentrieren sich die Hersteller auf die Produktion kompakter, automatisierter und hochempfindlicher Testsysteme, die auf Labore mit hohem Durchsatz zugeschnitten sind. Dies verändert die Erwartungen an Effizienz, Compliance und Skalierbarkeit in Qualitätskontrollumgebungen.
Marktdynamik für Endotoxin- und Pyrogentests
Treiber
„Steigender Bedarf aufgrund steigender Kontaminationsrisiken und strenger Vorschriften“
- Die zunehmende Verbreitung von Kontaminationsrisiken in der Pharma-, Biotechnologie- und Medizinprodukteproduktion, gepaart mit strengeren regulatorischen Rahmenbedingungen, ist ein wesentlicher Treiber für die erhöhte Nachfrage nach Endotoxin- und Pyrogen-Testlösungen
- So kündigte beispielsweise Onity, Inc. (Honeywell International, Inc.) im April 2024 Fortschritte in der biopharmazeutischen Sicherheitstechnologie an, die auf die Integration von Echtzeit-Erkennungssensoren in aseptische Produktionsumgebungen abzielen. Solche Entwicklungen wichtiger Akteure dürften das Wachstum des Marktes für Endotoxin- und Pyrogentests im asiatisch-pazifischen Raum ankurbeln.
- Da die Patientensicherheit und die Einhaltung gesetzlicher Vorschriften für Unternehmen oberste Priorität haben, werden Tests wie der Limulus-Amöbozytenlysat-Test (LAL) und der Monozytenaktivierungstest (MAT) zunehmend eingesetzt, da sie in der Lage sind, geringe Endotoxinwerte in injizierbaren Medikamenten, Impfstoffen und implantierbaren Geräten nachzuweisen.
- Darüber hinaus steigert das Wachstum von Biologika und personalisierten Medikamenten den Bedarf an zuverlässigen Hochdurchsatz-Testmethoden, die sich nahtlos in Qualitätssicherungsprozesse integrieren lassen. Automatisierte Testplattformen und Schnelltests werden zu kritischen Komponenten moderner Produktionslinien.
- Der Komfort der Automatisierung, die Reduzierung manueller Fehler und die Fähigkeit, große Testvolumina präzise durchzuführen, sind wichtige Faktoren, die die Einführung dieser Lösungen bei Pharmaunternehmen, Auftragsherstellern (CMOs) und Forschungslaboren vorantreiben. Der Trend zur Dezentralisierung der Produktion und zum Ausbau regionaler Biotech-Zentren trägt zusätzlich zum Marktwachstum im asiatisch-pazifischen Raum bei.
Einschränkung/Herausforderung
„ Bedenken hinsichtlich hoher Kosten und regulatorischer Harmonisierung “
- Die hohen Kosten für rekombinante oder alternative Endotoxin-Nachweissysteme sowie der Bedarf an Spezialinstrumenten können für kleine und mittlere Unternehmen in Schwellenländern im asiatisch-pazifischen Raum ein Hindernis darstellen.
- Während beispielsweise herkömmliche Gel-Clot-LAL-Tests relativ kostengünstig sind, sind fortschrittlichere kinetische chromogene und turbidimetrische Methoden sowie MAT-basierte Systeme mit deutlich höheren Anschaffungskosten und Schulungsanforderungen verbunden.
- Darüber hinaus führt die uneinheitliche Umsetzung von Vorschriften in den Ländern des asiatisch-pazifischen Raums – beispielsweise unterschiedliche Akzeptanzniveaus von rFC- oder MAT-Methoden – zu Unsicherheit bei Herstellern, die ihre Validierungsprozesse regional rationalisieren möchten.
- Die Bewältigung dieser Herausforderungen durch Kostenoptimierung, regionale Harmonisierung der Vorschriften und erweiterte Schulungsinitiativen ist entscheidend für die Förderung der Akzeptanz. Führende Akteure bieten zunehmend gebündelte Hardware-Software-Lösungen und technischen Support an, um diese Markteintrittsbarrieren zu verringern und ein nachhaltiges Marktwachstum zu fördern.
Marktumfang für Endotoxin- und Pyrogentests
Der Markt ist nach Produkten und Dienstleistungen, Testtyp, Typ, Produktkategorie, Form, Anwendung, Methode, Kaufart, Endprodukt und Endbenutzer segmentiert.
• Nach Produkten und Dienstleistungen
Der Markt für Endotoxin- und Pyrogentests ist nach Produkten und Dienstleistungen segmentiert in Detektionskits und Reagenzien, Instrumente und Systeme, Endotoxin- und Pyrogentestdienstleistungen sowie Verbrauchsmaterialien und Zubehör. Das Segment Detektionskits und Reagenzien dominierte den Markt mit dem größten Umsatzanteil von 46,8 % im Jahr 2024. Dies ist auf die hohe Nachfrage in der Pharma- und Biotechnologiebranche aufgrund der einfachen Handhabung und der zuverlässigen Leistung beim Endotoxinnachweis zurückzuführen.
Das Segment der Endotoxin- und Pyrogentestdienste wird aufgrund des zunehmenden Outsourcings durch Pharma- und Biotechunternehmen von 2025 bis 2032 voraussichtlich mit einer durchschnittlichen jährlichen Wachstumsrate von 11,2 % wachsen.
• Nach Testtyp
Der Markt für Endotoxin- und Pyrogentests ist nach Testtyp in Limulus-Amöbozytenlysat (LAL)-Test, TAL-Tests, Monozytenaktivierungstest (MAT), rekombinanten Faktor-C-Test (rFC), In-vitro-Test und Kaninchen-Pyrogentest unterteilt. Das LAL-Testsegment hielt im Jahr 2024 aufgrund seiner regulatorischen Akzeptanz und hohen Sensitivität mit 41,2 % den größten Marktanteil.
Das Segment der rekombinanten Faktor-C-Tests (rFC) wird voraussichtlich von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 12,7 % wachsen, was auf die Nachfrage nach tierfreien Tests und Nachhaltigkeitsaspekte zurückzuführen ist.
• Nach Typ
Der Markt für Endotoxin- und Pyrogentests ist nach Typ in vorgefertigte Endotoxin- und Pyrogentests, Proendotoxin- und Pyrogentests sowie kombinierte Endotoxin- und Pyrogentests unterteilt. Das Segment der kombinierten Endotoxin- und Pyrogentests hatte im Jahr 2024 mit 38,5 % den größten Marktanteil, da es mehrere Verunreinigungen effizient erkennen kann.
Das Segment der Proendotoxin- und Pyrogentests dürfte zwischen 2025 und 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 10,6 % wachsen, was auf die Nachfrage nach prädiktiven Tests im Frühstadium zurückzuführen ist.
• Nach Produktkategorie
Der Markt für Endotoxin- und Pyrogentests ist nach Produktkategorien in Clean Labeled Ingredient und konventionelle Tests unterteilt. Das konventionelle Segment führte den Markt mit einem Marktanteil von 58,1 % im Jahr 2024 an, da es weit verbreitete Reagenzien und Systeme umfasst.
Das Segment der Clean Label-Zutaten wird aufgrund von durch Transparenz bedingten regulatorischen Veränderungen und Verbraucherpräferenzen von 2025 bis 2032 voraussichtlich mit einer durchschnittlichen jährlichen Wachstumsrate von 9,3 % wachsen.
• Nach Formular
Der Markt für Endotoxin- und Pyrogentests ist in Pulver- und Flüssigkeitstests unterteilt. Das Flüssigkeitssegment hatte im Jahr 2024 aufgrund der einfachen Automatisierung und der direkten Nutzung den dominierenden Anteil von 63,9 %.
Das Pulversegment dürfte von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 8,8 % wachsen und profitiert dabei von der längeren Haltbarkeit und Transportierbarkeit.
• Nach Anwendung
Der Markt für Endotoxin- und Pyrogentests ist nach Anwendung in die Bereiche Arzneimittelherstellung, Medizinprodukteherstellung, Rohstoffproduktion und Verpackungsherstellung unterteilt. Aufgrund der hohen Anforderungen an die Sterilität hatte die Arzneimittelherstellung im Jahr 2024 mit 49,5 % den größten Anteil.
Aufgrund zunehmender behördlicher Prüfungen von Implantaten und chirurgischen Geräten wird für den Bereich der Herstellung medizinischer Geräte von 2025 bis 2032 ein durchschnittliches jährliches Wachstum von 10,1 % prognostiziert.
• Nach Methode
Der Markt für Endotoxin- und Pyrogentests ist methodisch in Gel-Clot-Endotoxin- und Pyrogentests, chromogene Endotoxin- und Pyrogentests und turbidimetrische Endotoxin- und Pyrogentests unterteilt. Die Gel-Clot-Methode dominierte den Markt mit einem Marktanteil von 42,7 % im Jahr 2024 aufgrund der Kosteneffizienz und der behördlichen Zulassung.
Es wird erwartet, dass die chromogene Methode zwischen 2025 und 2032 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 11,4 % am schnellsten wachsen wird, was auf ihre quantitative Genauigkeit und Automatisierungskompatibilität zurückzuführen ist.
• Nach Kaufart
Der Markt für Endotoxin- und Pyrogentests ist nach der Beschaffungsart in Großgruppen, mittlere und kleine Gruppen sowie Einzelpersonen segmentiert. Das Segment der Großgruppen hielt im Jahr 2024 mit 55,2 % den größten Marktanteil, was auf groß angelegte Beschaffungen durch Pharmaunternehmen und CDMOs zurückzuführen ist.
Das Segment der mittleren und kleinen Gruppen wird voraussichtlich zwischen 2025 und 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 9,9 % wachsen, wobei die Nachfrage von KMU und akademischen Einrichtungen steigt.
• Nach Endprodukt
Der Markt für Endotoxin- und Pyrogentests ist nach Endprodukt in Impfstoffe und/oder CGT, Biologika, Injektionspräparate und Sonstiges unterteilt. Das Segment Biologika hatte im Jahr 2024 mit 38,9 % den größten Anteil, getrieben durch die steigende Nachfrage nach monoklonalen Antikörpern und Biosimilars.
Aufgrund zunehmender Zulassungen für fortschrittliche Therapien und die Impfstoffentwicklung wird für das Impfstoff- und/oder CGT-Segment von 2025 bis 2032 ein durchschnittliches jährliches Wachstum von 12,1 % erwartet.
• Durch Endbenutzer
Der Markt für Endotoxin- und Pyrogentests ist nach Endverbrauchern segmentiert: Pharmaunternehmen, Biotechnologieunternehmen, Biomedizinunternehmen, Medizinproduktehersteller, Auftragsforschungsinstitute (CROs), Auftragsfertigungsunternehmen (CMOs) und weitere. Das Segment der Pharmaunternehmen dominierte den Markt mit einem Anteil von 40,4 % im Jahr 2024 aufgrund konsequenter Investitionen in Qualitätskontrolle und Konformitätsprüfungen.
Das Segment der Auftragsforschungsinstitute (CROs) dürfte zwischen 2025 und 2032 mit 11,6 % die höchste durchschnittliche jährliche Wachstumsrate (CAGR) verzeichnen, angetrieben durch zunehmendes Outsourcing und wachsende Arzneimittelforschungspipelines.
Regionale Analyse des Endotoxin- und Pyrogentests-Marktes
- Nordamerika dominierte den globalen Markt für Endotoxin- und Pyrogentests mit dem größten Umsatzanteil von 40,01 % im Jahr 2024, getrieben durch zunehmende regulatorische Strenge, starke biopharmazeutische Produktionspipelines und die weit verbreitete Verwendung von injizierbaren Medikamenten und Biologika
- Die Region profitiert von einer fortschrittlichen Gesundheitsinfrastruktur, einer hohen Anzahl klinischer Studien und einem zunehmenden Trend zu rekombinanten und tierversuchsfreien Testmethoden. Führende Unternehmen in den USA und Kanada investieren in automatisierte Endotoxin-Nachweissysteme und nachhaltige Testlösungen.
- Die wachsende Präferenz für schnellere und konforme Qualitätskontrollprotokolle stärkt die Marktpräsenz in dieser Region weiter
Einblicke in den Markt für Endotoxin- und Pyrogentests in den USA
Der US-Markt für Endotoxin- und Pyrogentests machte 2024 83 % des nordamerikanischen Marktanteils aus. Das Land ist führend aufgrund seiner groß angelegten Produktion von Biologika und Impfstoffen, hoher Investitionen in die pharmazeutische Forschung und Entwicklung sowie der schnellen Einführung von rekombinanten Faktor-C-Tests (rFC) und Monozytenaktivierungstests (MAT). Die regulatorische Anpassung an die Bemühungen der FDA, Alternativen zu Tierversuchen zu finden, beschleunigt das Marktwachstum zusätzlich.
Einblicke in den europäischen Markt für Endotoxin- und Pyrogentests
Der europäische Markt für Endotoxin- und Pyrogentests wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen. Dies ist auf das steigende Bewusstsein für Produktsicherheit, strenge Anforderungen des EU-Arzneibuchs und die zunehmende Zulassung biologischer Produkte zurückzuführen. Länder wie Deutschland, Großbritannien und Frankreich setzen verstärkt auf nachhaltige Testalternativen. Starkes Wachstum in der Auftragsfertigung und im F&E-Outsourcing trägt ebenfalls zur steigenden Marktnachfrage in der Arzneimittelentwicklung und der Medizinprodukteprüfung bei.
Einblicke in den britischen Markt für Endotoxin- und Pyrogentests
Der britische Markt für Endotoxin- und Pyrogentests dürfte im Prognosezeitraum stetig wachsen. Dies wird durch die an globale GMP-Standards angepassten MHRA-Vorschriften, einen reifen Biopharmasektor und steigende Investitionen in die klinische Forschungsinfrastruktur unterstützt. Die Nachfrage nach schnellen Endotoxin-Testmethoden zur Sterilitätssicherung und zur Herstellung injizierbarer Arzneimittel ist ein wichtiger Wachstumstreiber.
Markteinblick in Endotoxin- und Pyrogentests in Deutschland
Der deutsche Markt für Endotoxin- und Pyrogentests wird voraussichtlich aufgrund seiner robusten pharmazeutischen Produktionsbasis, der Automatisierung in Qualitätskontrolllaboren und der Unterstützung rekombinanter Testtechnologien ein deutliches Wachstum verzeichnen. Die Einhaltung gesetzlicher Vorschriften und ein starker Fokus auf Produktsicherheit und -effizienz prägen die Beschaffungstrends.
Markteinblicke für Endotoxin- und Pyrogentests im asiatisch-pazifischen Raum
Der Markt für Endotoxin- und Pyrogentests im asiatisch-pazifischen Raum wird voraussichtlich mit einer durchschnittlichen jährlichen Wachstumsrate von 24,02 % (2025–2032) wachsen. Dies ist auf steigende Pharmaexporte, die steigende Nachfrage nach Biologika und die staatliche Förderung der lokalen Arzneimittelproduktion in Ländern wie China, Japan und Indien zurückzuführen. Die rasante Ausweitung klinischer Studien und das gestiegene Bewusstsein für Kontaminationskontrollstandards fördern die Einführung von MAT- und rFC-Tests in der Region.
Einblicke in den japanischen Markt für Endotoxin- und Pyrogentests
Der japanische Markt für Endotoxin- und Pyrogentests verzeichnet aufgrund der hohen Innovationskraft im Bereich Zell- und Gentherapien und der gut regulierten Pharmabranche eine steigende Nachfrage nach Pyrogen- und Endotoxintests. Regulierungsbehörden fördern tierversuchsfreie Testalternativen, während die alternde Bevölkerung des Landes den Bedarf an injizierbaren Medikamenten erhöht.
Einblicke in den Markt für Endotoxin- und Pyrogentests in China
Der chinesische Markt für Endotoxin- und Pyrogentests eroberte 2024 den größten Marktanteil im asiatisch-pazifischen Raum. Dies ist auf die enorme pharmazeutische Produktionskapazität, die starke staatliche Unterstützung der lokalen Biologikaproduktion und die zunehmende Einführung nachhaltiger Qualitätskontrolllösungen zurückzuführen. Der Aufstieg inländischer Anbieter, die kostengünstige Testkits und -dienstleistungen anbieten, treibt das Marktwachstum weiter voran.
Marktanteile für Endotoxin- und Pyrogentests
Die Endotoxin- und Pyrogentestbranche wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Pall Corporation (USA)
- Thermo Fisher Scientific Inc. (USA)
- Charles River Laboratories (USA)
- Eurofins Scientific (Luxemburg)
- SGS Société Générale de Surveillance SA (Schweiz)
- Lonza (Schweiz)
- Merck KGaA (Deutschland)
- STERIS (Irland)
- Sartorius AG (Deutschland)
- BIOMÉRIEUX (Frankreich)
- Ellab A/S (Dänemark)
- ASSOCIATES OF CAPE COD, INC. (USA)
- WuXi AppTec (China)
- Microcoat Biotechnologie GmbH (Deutschland)
Neueste Entwicklungen auf dem globalen Markt für Endotoxin- und Pyrogentests
- Im März 2024 gab die Lonza Group den Ausbau ihrer Endotoxin- und Pyrogentestkapazitäten durch die Einführung eines neuen Testkits auf Basis von rekombinantem Faktor C (rFC) bekannt. Diese Entwicklung steht im Einklang mit der zunehmenden regulatorischen Akzeptanz tierversuchsfreier Testmethoden und unterstützt Nachhaltigkeitsziele durch die Reduzierung der Abhängigkeit von Pfeilschwanzkrebsblut.
- Im Februar 2024 führte Charles River Laboratories verbesserte Monozytenaktivierungstest-Plattformen (MAT) durch Automatisierungsintegration ein, um den Durchsatz und die Reproduzierbarkeit der Pyrogenerkennung in Biologika und Zelltherapien zu verbessern. Die Innovation zielt darauf ab, die Arbeitsabläufe von Pharmaunternehmen zu optimieren, die strenge globale Vorschriften einhalten müssen.
- Im Januar 2024 erweiterte die FUJIFILM Wako Chemicals USA Corporation ihr Limulus-Amöbozytenlysat (LAL)-Reagenzienportfolio mit erhöhter Sensitivität und reduzierter Variabilität und verbesserte so die Zuverlässigkeit von Endotoxintests in parenteralen Arzneimitteln und Medizinprodukten. Das Unternehmen kündigte außerdem neue Vertriebspartnerschaften an, um seine Reichweite in Südostasien zu erweitern.
- Im Dezember 2023 erhielt Associates of Cape Cod, Inc. (ACC) weitere Zulassungen von asiatischen Aufsichtsbehörden für seinen PyroSmart NextGen rFC-Test. Die Zulassung soll die Einführung nachhaltiger, tierversuchsfreier Endotoxin-Testmethoden bei Biopharma-Herstellern in Japan, China und Südkorea beschleunigen.
- Im Oktober 2023 stellte Merck KGaA (MilliporeSigma) eine neue Hochdurchsatz-Lösung für turbidimetrische Endotoxintests vor, die für die Impfstoff- und Biologikaproduktion entwickelt wurde. Die Markteinführung ist Teil der Initiative des Unternehmens, schnellere Chargenfreigabetests zu ermöglichen und gleichzeitig die sich entwickelnden GMP-Standards zu erfüllen.
- Im September 2023 gab Thermo Fisher Scientific die Entwicklung einer integrierten LAL-Testautomatisierungsplattform in Zusammenarbeit mit globalen CDMOs bekannt. Diese Lösung kombiniert LAL-Tests mit Echtzeit-Datenerfassung und zielt darauf ab, menschliche Fehler zu reduzieren, die Effizienz zu verbessern und die Auditbereitschaft bei Zulassungsanträgen sicherzustellen.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 COST ANALYSIS BREAKDOWN
8 TECHNONLOGY ROADMAP
9 INNOVATION TRACKER AND STRATEGIC ANALYSIS
9.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
9.1.1 JOINT VENTURES
9.1.2 MERGERS AND ACQUISITIONS
9.1.3 LICENSING AND PARTNERSHIP
9.1.4 TECHNOLOGY COLLABORATIONS
9.1.5 STRATEGIC DIVESTMENTS
9.2 NUMBER OF PRODUCTS IN DEVELOPMENT
9.3 STAGE OF DEVELOPMENT
9.4 TIMELINES AND MILESTONES
9.5 INNOVATION STRATEGIES AND METHODOLOGIES
9.6 RISK ASSESSMENT AND MITIGATION
9.7 FUTURE OUTLOOK
10 REGULATORY COMPLIANCE
10.1 REGULATORY AUTHORITIES
10.2 REGULATORY CLASSIFICATIONS
10.2.1 CLASS I
10.2.2 CLASS II
10.2.3 CLASS III
10.3 REGULATORY SUBMISSIONS
10.4 INTERNATIONAL HARMONIZATION
10.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
10.6 REGULATORY CHALLENGES AND STRATEGIES
11 REIMBURSEMENT FRAMEWORK
12 VALUE CHAIN ANALYSIS
13 HEALTHCARE ECONOMY
13.1 HEALTHCARE EXPENDITURE
13.2 CAPITAL EXPENDITURE
13.3 CAPEX TRENDS
13.4 CAPEX ALLOCATION
13.5 FUNDING SOURCES
13.6 INDUSTRY BENCHMARKS
13.7 GDP RATION IN OVERALL GDP
13.8 HEALTHCARE SYSTEM STRUCTURE
13.9 GOVERNMENT POLICIES
13.1 ECONOMIC DEVELOPMENT
14 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY PRODUCT AND SERVICES
14.1 OVERVIEW
14.2 DETECTION KITS & REAGENTS
14.2.1 LAL TEST REAGENTS
14.2.2 MYCOPLASMA DETECTION & REMOVAL
14.2.3 PCR MYCOPLASMA DETECTION KIT
14.2.4 MYCOPLASMA ELIMINATION COCKTAIL
14.2.5 OTHERS
14.3 INSTRUMENTS & SYSTEMS
14.3.1 SERIES TUBE READER
14.3.2 MICROPLATE READER
14.3.3 LOW ENDOTOXIN RECOVERY (LER)
14.3.4 ENDOTOXIN REMOVAL
14.3.5 LOW ENDOTOXIN RECOVERY (LER)
14.3.6 OTHERS
14.4 CONSUMABLES & ACCESSORIES
14.5 SOFTWARE AND SERVICES
14.6 OTHERS
15 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY TEST TYPE
15.1 OVERVIEW
15.2 LIMULUS AMOEBOCYTE LYSATE (LAL) TEST
15.3 MONOCYTE ACTIVATION TEST (MAT) TEST
15.4 RECOMBINANT FACTOR C (RFC) ASSAY
15.5 RABBIT PYROGEN TEST
16 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY TYPE
16.1 OVERVIEW
16.2 PRE-FORMED ENDOTOXIN AND PYROGEN TESTING
16.3 PROENDOTOXIN AND PYROGEN TESTING
16.4 COMBINE ENDOTOXIN AND PYROGEN TESTING
17 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY FORM
17.1 OVERVIEW
17.2 POWDER
17.3 LIQUID
18 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY PRODUCT CATEGORY
18.1 OVERVIEW
18.2 CLEAN LABELED INGREDIENT
18.3 CONVENTIONAL
19 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 PHARMACEUTICAL MANUFACTURING
19.3 MEDICAL DEVICE MANUFACTURING
19.4 RAW MATERIALS PRODUCTION
19.5 PACKAGING MANUFACTURE
20 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY METHOD
20.1 OVERVIEW
20.2 GEL CLOT ENDOTOXIN AND PYROGEN TEST
20.3 CHROMOGENIC ENDOTOXIN AND PYROGEN TEST
20.4 TURBIDIMETRIC ENDOTOXIN AND PYROGEN TEST
21 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY MODE OF PURCHASE
21.1 OVERVIEW
21.2 LARGE GROUP
21.3 MID AND SMALL GROUP
21.4 INDIVIDUAL
22 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY END PRODUCT
22.1 OVERVIEW
22.2 BIOLOGICS
22.3 VACCINES AND/ OR CGT
22.4 INJECTABLES
22.5 OTHERS
22.5.1 ENDOSCOPES
22.5.2 REUSABLE BIOMEDICAL DEVICES
22.5.3 OTHERS
23 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY END USER
23.1 OVERVIEW
23.2 PHARMACEUTICAL COMPANIES
23.3 BIOTECHNOLOGY COMPANIES
23.4 BIOMEDICAL COMPANIES
23.5 MEDICAL DEVICE COMPANIES
23.6 CONTRACT RESEARCH ORGANIZATION (CRO)
23.7 CONTRACT MANUFACTURING ORGANIZATION (CMO)
24 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, GEOGRAPHY
24.1 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.2 NORTH AMERICA
24.2.1 U.S.
24.2.2 CANADA
24.2.3 MEXICO
24.3 EUROPE
24.3.1 GERMANY
24.3.2 FRANCE
24.3.3 U.K.
24.3.4 ITALY
24.3.5 SPAIN
24.3.6 RUSSIA
24.3.7 BELGIUM
24.3.8 NETHERLANDS
24.3.9 SWITZERLAND
24.3.10 REST OF EUROPE
24.4 ASIA-PACIFIC
24.4.1 JAPAN
24.4.2 CHINA
24.4.3 SOUTH KOREA
24.4.4 INDIA
24.4.5 AUSTRALIA
24.4.6 SINGAPORE
24.4.7 MALAYSIA
24.4.8 REST OF ASIA-PACIFIC
24.5 SOUTH AMERICA
24.5.1 BRAZIL
24.5.2 ARGENTINA
24.5.3 REST OF SOUTH AMERICA
24.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, COMPANY PROFILE
27.1 PALL EUROPE LIMITED (DANAHER CORPORATION)
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 THERMO FISHER SCIENTIFIC INC.
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 CHARLES RIVER LABORATORIES
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 EUROFINS SCIENTIFIC
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 SGS SOCIÉTÉ GÉNÉRALE DE SURVEILLANCE SA.
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 PACIFIC BIOLABS
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 LONZA
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 MERCK KGAA
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 STERIS
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 SARTORIUS AG
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 BIOMÉRIEUX
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 FUJIFILM WAKO PURE CHEMICAL CORPORATION
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 ELLAB A/S.
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 ASSOCIATES OF CAPE COD, INC (SEIKAGAKU CORPORATION)
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 WUXI APPTEC
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 GENSCRIPT
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 MICROCOAT BIOTECHNOLOGIE GMBH
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 SANQUIN
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 READING SCIENTIFIC SERVICES LTD
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.2 NANOCOMPOSIX
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 ZWISLER LABORATORIUM GMBH
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 NELSON LABORATORIES, LLC – A SOTERA HEALTH COMPANY
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 NORTH AMERICAN SCIENCE ASSOCIATES, LLC
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 PROMEGA CORPORATION
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 HYCULT BIOTECH INC.
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 ALMAC GROUP
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 MAT BIOTECH
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
27.28 SOLVIAS
27.28.1 COMPANY OVERVIEW
27.28.2 REVENUE ANALYSIS
27.28.3 GEOGRAPHIC PRESENCE
27.28.4 PRODUCT PORTFOLIO
27.28.5 RECENT DEVELOPMENTS
27.29 WICKHAM MICRO LIMITED
27.29.1 COMPANY OVERVIEW
27.29.2 REVENUE ANALYSIS
27.29.3 GEOGRAPHIC PRESENCE
27.29.4 PRODUCT PORTFOLIO
27.29.5 RECENT DEVELOPMENTS
27.3 CREATIVE BIOLABS
27.30.1 COMPANY OVERVIEW
27.30.2 REVENUE ANALYSIS
27.30.3 GEOGRAPHIC PRESENCE
27.30.4 PRODUCT PORTFOLIO
27.30.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
28 RELATED REPORTS
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.