Global Electronic Clinical Outcome Assessment Ecoa Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1.70 Billion
USD
5.52 Billion
2024
2032
USD
1.70 Billion
USD
5.52 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 5.52 Billion | |
|
|
|
|
Globale Marktsegmentierung für elektronische klinische Ergebnisbewertungen (eCOA) nach Typ (Clinician Reported Outcome Assessment (CLINRO), Patient Reported Outcome Assessment (PRO), Observer Reported Outcome Assessment (OBSRO) und Performance Outcome Assessment (PERFO)), Modalität (standortbasierte Lösungen, Weblösungen und Handheld), Endbenutzer (Auftragsforschungsinstitute (CROs), Pharma- und Biotechnologieunternehmen, Medizinproduktehersteller, Krankenhäuser/Gesundheitsdienstleister, Beratungsunternehmen, akademische und Forschungsinstitute und andere), Bereitstellungsmodus (Cloud-basiert und webgehostet) – Branchentrends und Prognose bis 2032
Marktgröße für elektronische klinische Ergebnisbewertung (eCOA)
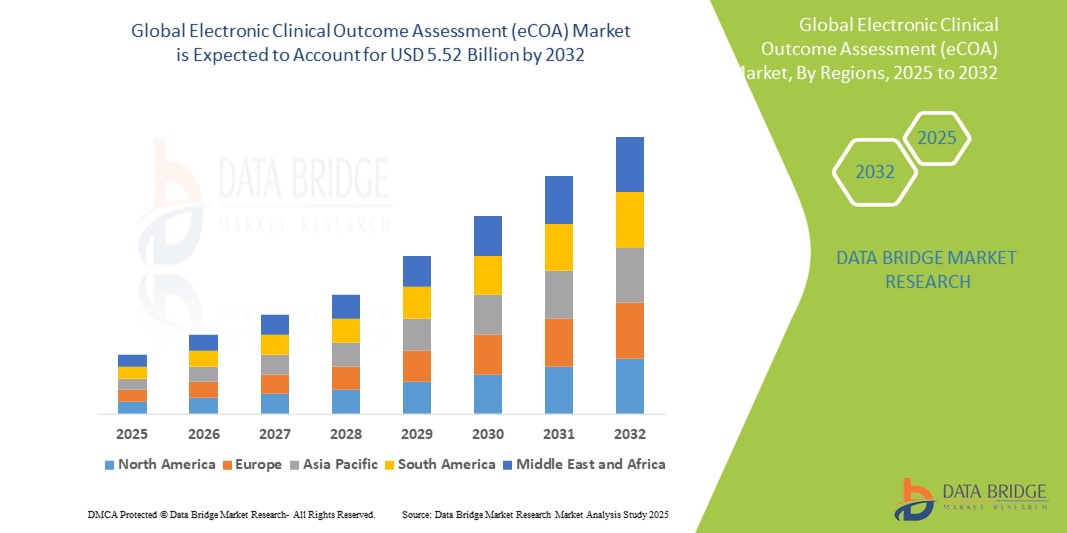
- Der globale Markt für elektronische klinische Ergebnisbewertungen (eCOA) wurde im Jahr 2024 auf 1,70 Milliarden US-Dollar geschätzt und soll bis 2032 5,52 Milliarden US-Dollar erreichen , bei einer CAGR von 15,80 % im Prognosezeitraum.
- Das Marktwachstum wird vor allem durch die zunehmende Nutzung digitaler Technologien in klinischen Studien und der Gesundheitsforschung vorangetrieben, die eine genauere und effizientere Erfassung und Überwachung von Patientendaten ermöglichen.
- Darüber hinaus treibt die steigende Nachfrage nach Echtzeit-Patienteninformationen, verbesserter Einhaltung gesetzlicher Vorschriften und verbesserter Datenintegrität die Einführung von eCOA-Lösungen bei Pharmaunternehmen, Auftragsforschungsinstituten (CROs) und Gesundheitsdienstleistern voran.
Marktanalyse für elektronische klinische Ergebnisbewertung (eCOA)
- eCOA-Lösungen, die die elektronische Erfassung klinischer Ergebnisdaten direkt von Patienten, Pflegepersonal oder Ärzten ermöglichen, sind aufgrund ihrer verbesserten Datengenauigkeit, Echtzeit-Überwachungsmöglichkeiten und nahtlosen Integration in digitale Gesundheitsökosysteme zunehmend wichtige Bestandteile moderner klinischer Studien und der Gesundheitsforschung.
- Die steigende Nachfrage nach eCOA wird vor allem durch die weitverbreitete Nutzung digitaler Gesundheitstechnologien, die zunehmende Betonung patientenzentrierter Studien und die zunehmende Präferenz für benutzerfreundliche Methoden zur Ferndatenerfassung, die die Effizienz und Compliance von Studien verbessern, vorangetrieben.
- Nordamerika dominiert den Markt für elektronische klinische Ergebnisbewertungen (eCOA) mit dem größten Umsatzanteil von 43,5 % im Jahr 2024. Dies ist gekennzeichnet durch die frühe Einführung digitaler Lösungen für klinische Studien, starke Pharma- und Biotech-Sektoren sowie regulatorische Rahmenbedingungen, die die elektronische Datenerfassung unterstützen. Die USA verzeichnen ein beträchtliches Wachstum, das durch Innovationen sowohl etablierter Anbieter als auch aufstrebender Technologieanbieter mit Schwerpunkt auf mobilen und Cloud-basierten Plattformen vorangetrieben wird.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum die am schnellsten wachsende Region im Markt für elektronische klinische Ergebnisbewertungen (eCOA) sein. Dies ist auf die zunehmenden Aktivitäten im Bereich klinischer Studien, steigende Investitionen im Gesundheitswesen und das wachsende Bewusstsein für die Vorteile digitaler Tools in Schwellenländern wie China und Indien zurückzuführen.
- Das Segment „Patient Reported Outcome Assessment“ (PRO) dominiert den Markt für elektronische klinische Ergebnisbewertungen (eCOA) mit einem Marktanteil von 48,5 % im Jahr 2024. Grund dafür ist seine entscheidende Rolle bei der Erfassung der Patientenperspektiven hinsichtlich der Wirksamkeit und Lebensqualität der Behandlung, die von Sponsoren und Aufsichtsbehörden zunehmend priorisiert werden.
Berichtsumfang und Marktsegmentierung für elektronische klinische Ergebnisbewertung (eCOA)
|
Eigenschaften |
Wichtige Markteinblicke zur elektronischen klinischen Ergebnisbewertung (eCOA) |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für die elektronische klinische Ergebnisbewertung (eCOA)
„Verbesserte Effizienz klinischer Studien durch KI und Fernüberwachung von Patienten“
- Ein bedeutender und sich beschleunigender Trend im globalen eCOA-Markt ist die zunehmende Integration von künstlicher Intelligenz (KI) und Technologien zur Patientenfernüberwachung in Plattformen zur Datenerfassung für klinische Studien. Diese Technologiefusion verbessert die Genauigkeit, Aktualität und Patientenzentrierung klinischer Ergebnisbewertungen erheblich.
- Führende eCOA-Anbieter wie Medidata und ERT nutzen beispielsweise KI-gestützte Analysen, um Muster in Patientendaten zu erkennen. Dies ermöglicht eine frühzeitige Erkennung von Nebenwirkungen und eine verbesserte Entscheidungsfindung bei Studien. Ebenso ermöglichen tragbare Geräte in Verbindung mit eCOA-Plattformen eine kontinuierliche Echtzeitüberwachung der Patientengesundheitsdaten über herkömmliche Vor-Ort-Besuche hinaus.
- Die KI-Integration in eCOA ermöglicht Funktionen wie prädiktive Analysen zur Patientenadhärenz, automatisierte Datenqualitätsprüfungen und intelligente Warnmeldungen bei ungewöhnlichen Patientenreaktionen. Darüber hinaus bieten Fernüberwachungsfunktionen den Patienten komfortable, benutzerfreundliche Schnittstellen, um Ergebnisse von zu Hause aus zu melden, was die Datenvollständigkeit und das Engagement verbessert.
- Die nahtlose Integration von eCOA-Systemen in umfassendere digitale Gesundheits- und klinische Studienmanagementplattformen ermöglicht es Sponsoren, das Datenmanagement zu zentralisieren und Studienabläufe zu optimieren. Über einheitliche Dashboards können klinische Teams Patientendaten, die Leistung der Standorte und die Einhaltung gesetzlicher Vorschriften in Echtzeit überwachen.
- Dieser Trend zu intelligenteren, vernetzten und patientenfreundlicheren Lösungen für klinische Ergebnisse verändert die Erwartungen an die Datenerfassung in klinischen Studien grundlegend. Unternehmen wie Oracle Health und CRF Health entwickeln daher KI-gestützte eCOA-Plattformen mit verbesserten Prognosefunktionen und Funktionen zur Ferndatenerfassung.
- Die Nachfrage nach eCOA-Lösungen mit KI und integrierter Patientenfernüberwachung wächst in den Bereichen Pharma, Biotechnologie und Medizintechnik rasant, da die Interessengruppen zunehmend Wert auf Studieneffizienz, Datengenauigkeit und Patientenerfahrung legen.
Marktdynamik für elektronische klinische Ergebnisbewertung (eCOA)
Treiber
„Steigende Nachfrage nach patientenzentrierten Studien und digitaler Datengenauigkeit“
- Der zunehmende Fokus auf patientenzentrierte klinische Studien, kombiniert mit dem wachsenden Bedarf an präziser, digitaler Datenerfassung in Echtzeit, ist ein wesentlicher Treiber für die erhöhte Nachfrage nach Lösungen zur elektronischen klinischen Ergebnisbewertung (eCOA).
- So führte Medidata, ein Unternehmen von Dassault Systèmes, im Januar 2024 neue KI-gestützte Erweiterungen seiner eCOA-Plattform ein, um die Patienten-Compliance und die Datenqualität in dezentralen Studien zu verbessern. Solche Innovationen wichtiger Branchenakteure dürften das Wachstum des eCOA-Marktes im Prognosezeitraum vorantreiben.
- Pharma- und Biotechnologieunternehmen versuchen, klinische Studienprozesse zu rationalisieren und die Markteinführungszeit zu verkürzen. eCOA-Plattformen bieten erweiterte Funktionen wie Echtzeit-Datenerfassung, Ferndiagnose von Patienten und automatisierte Validierung und stellen damit eine wesentliche Verbesserung gegenüber herkömmlichen papierbasierten Methoden dar.
- Darüber hinaus positioniert sich eCOA durch die zunehmende Einführung dezentraler und hybrider Modelle für klinische Studien als wesentliche Komponente für die Ferndatenerfassung, verbessert die Patienteneinbindung und gewährleistet gleichzeitig hohe Standards der Einhaltung gesetzlicher Vorschriften.
- Die Fähigkeit von eCOA-Plattformen, die Studieneffizienz durch elektronische Datenerfassung, mehrsprachigen Support und die Integration mit tragbaren Geräten oder mobilen Anwendungen zu steigern, ist ein Schlüsselfaktor für ihre Verbreitung bei CROs, Pharmaunternehmen und Forschungseinrichtungen. Die zunehmende Bedeutung der Reduzierung der Abbruchraten klinischer Studien und der Verbesserung der Datenintegrität unterstützt die breite Integration von eCOA-Lösungen in die moderne klinische Forschung.
Einschränkung/Herausforderung
„Bedenken hinsichtlich Datenschutz, Einhaltung gesetzlicher Vorschriften und hoher Implementierungskosten“
- Bedenken hinsichtlich Datenschutz, Einhaltung gesetzlicher Vorschriften und die hohen anfänglichen Implementierungskosten von Plattformen zur elektronischen klinischen Ergebnisbewertung (eCOA) stellen erhebliche Herausforderungen für eine breitere Marktakzeptanz dar
- Da eCOA-Systeme die elektronische Erfassung und Übermittlung sensibler Patientendaten beinhalten, unterliegen sie strengen Datenschutzbestimmungen wie HIPAA, DSGVO und 21 CFR Part 11, was die Einhaltung der Vorschriften für Sponsoren und CROs komplex und ressourcenintensiv macht.
- So haben beispielsweise mehrere Sponsoren klinischer Studien Bedenken hinsichtlich der vollständigen Umstellung auf eCOA-Systeme geäußert, da Unsicherheiten hinsichtlich der Datenlokalisierungsregeln bestehen und die Sicherstellung der grenzüberschreitenden Datenkonformität, insbesondere bei Studien in mehreren Regionen, komplex ist.
- Die Bewältigung dieser Herausforderungen erfordert eine robuste Datensicherheitsinfrastruktur, regelmäßige Audits und die Einhaltung globaler Compliance-Standards. Führende eCOA-Anbieter wie Oracle Health und Signant Health investieren erheblich in verschlüsselte Plattformen und regulatorische Schulungen, um Risiken zu minimieren und das Vertrauen der Studienbeteiligten zu wahren.
- Darüber hinaus können die hohen Vorlaufkosten für die Einführung von eCOA-Systemen – einschließlich Lizenzgebühren, Hardwarebeschaffung, Mitarbeiterschulung und Systemintegration – insbesondere für kleine und mittelgroße Forschungseinrichtungen ein Hindernis darstellen. Obwohl langfristige Vorteile wie verbesserte Datengenauigkeit und verkürzte Studiendauer allgemein anerkannt sind, kann die anfängliche finanzielle Belastung die Einführung in ressourcenbeschränkten Einrichtungen behindern.
- Die Bewältigung dieser Herausforderungen durch skalierbare Preismodelle, Cloud-basierte Bereitstellung und kontinuierliche Innovation bei sicheren, benutzerfreundlichen Plattformen wird entscheidend sein, um eine breitere und nachhaltige Einführung von eCOA-Lösungen in der gesamten klinischen Forschung voranzutreiben.
Marktumfang für elektronische klinische Ergebnisbewertung (eCOA)
Der Markt ist nach Typ, Modalität, Endbenutzer und Liefermodus segmentiert
- Nach Typ
Der Markt für elektronische klinische Ergebnisbewertungen (eCOA) ist nach Typ segmentiert in patientenberichtete Ergebnisse (PRO), klinisch-beurteilte Ergebnisse (ClinRO), beobachterberichtete Ergebnisse (ObsRO) und Leistungsergebnisse (PerfO). Das Segment patientenberichtete Ergebnisse (PRO) hatte 2024 mit 48,5 % den größten Marktanteil, was auf seinen patientenzentrierten Ansatz zurückzuführen ist, der Einblicke aus erster Hand in die Erfahrungen, Symptome und Behandlungsergebnisse der Patienten liefert. PRO-Tools ermöglichen es Patienten, ihre Gesundheitsdaten in Echtzeit direkt über elektronische Plattformen zu melden. Dies erhöht die Datenpräzision und die Patientenbeteiligung und verbessert letztlich die Qualität klinischer Studien.
Das Segment der klinisch berichteten Ergebnisse (ClinRO) dürfte im Prognosezeitraum aufgrund der zunehmenden Komplexität klinischer Studien und des Bedarfs an präzisen und standardisierten Datenerhebungsmethoden deutlich wachsen. ClinRO umfasst Bewertungen durch geschultes medizinisches Fachpersonal und liefert objektive und zuverlässige Daten zur Bewertung klinischer Interventionen, insbesondere in Fällen, in denen eine Selbstauskunft der Patienten nicht möglich ist.
- Nach Modalität
Der Markt für elektronische klinische Ergebnisbewertungen (eCOA) ist nach Modalität in standortbasierte Lösungen, Weblösungen und Handheld-Geräte segmentiert. Das Segment Weblösungen hatte 2024 den größten Marktanteil, was auf benutzerfreundliche Oberflächen, einfache Zugänglichkeit und einen geringeren Investitionsbedarf zurückzuführen ist. Webgehostete Lösungen speichern Kundendaten auf Cloud-Servern, die über das Web mit einfacher Computerhardware und Internetverbindung zugänglich sind. Dies bietet Flexibilität bei der Anpassung und ermöglicht die maßgeschneiderte Lösung an spezifische Kundenbedürfnisse.
Das Segment der Handheld-Geräte wird im Prognosezeitraum voraussichtlich deutlich wachsen, getrieben durch den zunehmenden Einsatz mobiler Technologien in klinischen Studien. Handheld-Geräte ermöglichen die Echtzeit-Datenerfassung und verbessern die Patienten-Compliance, was sie zu einer attraktiven Option für dezentrale und Remote-Studien macht.
- Nach Endbenutzer
Der Markt für elektronische klinische Ergebnisbewertungen (eCOA) ist nach Endnutzern segmentiert in Pharma- und Biotechnologieunternehmen, Auftragsforschungsinstitute (CROs), Medizintechnikunternehmen, Krankenhäuser/Gesundheitsdienstleister, Beratungsunternehmen, Hochschulen und Forschungsinstitute und weitere. Das Segment der Pharma- und Biotechnologieunternehmen dominiert den Markt mit einem Marktanteil von 50,66 % im Jahr 2024. Diese Dominanz ist auf die entscheidende Rolle von eCOA-Lösungen bei der Optimierung der Datenerfassung und -analyse während der Arzneimittelentwicklung, der Gewährleistung der Einhaltung regulatorischer Standards und der Verbesserung der Genauigkeit klinischer Studiendaten zurückzuführen.
Das Segment der Auftragsforschungsinstitute (CROs) dürfte im Prognosezeitraum deutlich wachsen, getrieben durch den zunehmenden Trend großer Biopharma- und Medizintechnikunternehmen, das Management klinischer Forschung auszulagern. CROs bieten umfassende Dienstleistungen von der Studienplanung über die Patientenrekrutierung bis hin zur Datenerhebung und -analyse und sind damit ein integraler Bestandteil der eCOA-Landschaft.
Nach Liefermodus
Der Markt für elektronische klinische Ergebnisbewertungen (eCOA) ist nach Bereitstellungsart in Cloud-basierte und webbasierte Lösungen segmentiert. Das Segment der webbasierten Lösungen hatte im Jahr 2025 aufgrund seiner Kosteneffizienz im Vergleich zu Cloud-basierten Lösungen den größten Marktanteil von 58,9 %. Webbasierte Plattformen erfordern geringere Infrastrukturinvestitionen für Endnutzer und reduzieren so die Investitionsausgaben für Pharmaunternehmen, CROs und Gesundheitsdienstleister.
Das Segment der Cloud-Lösungen dürfte im Prognosezeitraum deutlich wachsen, getrieben durch Skalierbarkeit, Flexibilität und Kosteneffizienz. Cloud-basierte Plattformen ermöglichen den Beteiligten an klinischen Studien einen einfacheren und schnelleren Zugriff auf Daten, unabhängig von ihrem Standort, was bei Studien an mehreren Standorten entscheidend ist.
Regionale Marktanalyse für elektronische klinische Ergebnisbewertung (eCOA)
- Nordamerika dominiert den Markt für elektronische klinische Ergebnisbewertungen (eCOA) mit dem größten Umsatzanteil von 43,5 % im Jahr 2024, was auf die frühe Einführung digitaler Lösungen für klinische Studien, starke Pharma- und Biotech-Sektoren sowie regulatorische Rahmenbedingungen zur Unterstützung der elektronischen Datenerfassung zurückzuführen ist.
- Die Region profitiert von einem robusten regulatorischen Rahmen, der die digitale Transformation der klinischen Forschung unterstützt und Pharmaunternehmen und Auftragsforschungsinstitute ermutigt, eCOA-Plattformen einzuführen, um die Datengenauigkeit und die Einhaltung gesetzlicher Vorschriften zu verbessern.
- Darüber hinaus tragen hohe Investitionen in Forschung und Entwicklung, eine gut etablierte Gesundheitsinfrastruktur und die frühzeitige Einführung dezentraler und patientenzentrierter klinischer Studien maßgeblich zum Marktwachstum bei. Die Präsenz führender eCOA-Lösungsanbieter und CROs beschleunigt die regionale Verbreitung elektronischer Instrumente zur klinischen Ergebnisbewertung zusätzlich.
Markteinblick in die elektronische klinische Ergebnisbewertung (eCOA) in den USA
Der US-Markt für elektronische klinische Ergebnisbewertungen (eCOA) erzielte 2024 mit 79,6 % den größten Umsatzanteil in Nordamerika. Dies ist auf die führende Rolle des Landes bei klinischen Studien und die schnelle Digitalisierung klinischer Forschungspraktiken zurückzuführen. Regulierungsbehörden wie die FDA setzen sich nachdrücklich für den Einsatz digitaler Tools zur Verbesserung der Datenqualität und der Patienteneinbindung ein und tragen so zur breiten Akzeptanz von eCOA-Systemen bei. Darüber hinaus treibt der steigende Bedarf an dezentralen und hybriden klinischen Studienmodellen die Nachfrage nach Plattformen zur dezentralen Echtzeit-Patientendatenerfassung an. Der US-Markt profitiert zudem von einer starken F&E-Finanzierung, einer starken Präsenz großer Pharmakonzerne und einer hochentwickelten Gesundheits-IT-Infrastruktur.
Markteinblick in die elektronische klinische Ergebnisbewertung (eCOA) in Europa
Der europäische Markt für elektronische klinische Ergebnisbewertungen (eCOA) wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen. Dies wird durch die zunehmende regulatorische Betonung von Real-World-Evidenz, Patientenzentrierung und Datenstandardisierung in klinischen Studien vorangetrieben. Der gestiegene Bedarf an mehrsprachigen und kulturell angepassten digitalen Lösungen in der gesamten EU beschleunigt die Einführung flexibler, skalierbarer eCOA-Plattformen. Darüber hinaus unterstützt die Zunahme akademischer Forschungskooperationen in Verbindung mit einer positiven Politik für die digitale Gesundheitstransformation das regionale Wachstum. Länder wie Deutschland, Großbritannien und Frankreich sind führend bei der Technologieeinführung in ihren jeweiligen Studienökosystemen.
Markteinblick in die elektronische klinische Ergebnisbewertung (eCOA) in Großbritannien
Der britische Markt für elektronische klinische Ergebnisbewertungen (eCOA) wird im Prognosezeitraum voraussichtlich mit einer bemerkenswerten jährlichen Wachstumsrate wachsen, unterstützt durch den starken biopharmazeutischen Forschungs- und Entwicklungssektor und die proaktiven digitalen Gesundheitsstrategien des britischen Gesundheitsdienstes NHS. Die zunehmende Anzahl dezentraler Studien und die regulatorische Klarheit im Bereich der elektronischen Datenerfassung fördern die Marktakzeptanz. Dank einer fortschrittlichen klinischen Forschungslandschaft und erheblichen Investitionen in die Gesundheitsinformatik erlebt Großbritannien eine rasante Verbreitung von eCOA-Technologien, um Compliance sicherzustellen, die Patienteneinbindung zu verbessern und eine effiziente Ergebnisverfolgung zu ermöglichen.
Markteinblick in die elektronische klinische Ergebnisbewertung (eCOA) in Deutschland
Der deutsche Markt für elektronische klinische Ergebnisbewertungen (eCOA) wird im Prognosezeitraum voraussichtlich mit einer beträchtlichen jährlichen Wachstumsrate wachsen, was auf den Ruf Deutschlands für exzellente klinische Studien und strenge Datenschutzgesetze zurückzuführen ist. Deutsche Aufsichtsbehörden legen Wert auf die Zuverlässigkeit und Sicherheit klinischer Daten und veranlassen Sponsoren und CROs, in sichere, validierte eCOA-Lösungen zu investieren. Darüber hinaus fördern die wachsende Nachfrage nach Echtzeit-Datenerfassung in Phase-I-IV-Studien und die starke IT-Infrastruktur im Gesundheitswesen die weitere Integration von eCOA-Technologien in die Medizinprodukte- und Pharmaforschung.
Markteinblick in die elektronische klinische Ergebnisbewertung (eCOA) im asiatisch-pazifischen Raum
Der Markt für elektronische klinische Ergebnisbewertungen (eCOA) im asiatisch-pazifischen Raum wird im Prognosezeitraum 2025 bis 2032 voraussichtlich die höchste durchschnittliche jährliche Wachstumsrate (CAGR) aufweisen. Dies ist auf die zunehmende klinische Forschungsaktivität und die digitale Transformation des Gesundheitswesens in Ländern wie China, Indien, Südkorea und Japan zurückzuführen. Die Ausweitung multinationaler Studien und die Verfügbarkeit vielfältiger Patientenpopulationen unterstützen das regionale Wachstum. Staatliche Anreize für die Einführung digitaler Gesundheitsplattformen und die wachsende Nachfrage nach mobilen Lösungen machen die Einführung von eCOA in städtischen und halbstädtischen Regionen praktikabler und weitverbreiteter. Lokale Partnerschaften zwischen CROs und globalen Pharmaunternehmen treiben die Implementierung von eCOA weiter voran.
Markteinblick in Japan zur elektronischen klinischen Ergebnisbewertung (eCOA)
Der japanische Markt für elektronische klinische Ergebnisbewertungen (eCOA) gewinnt aufgrund der technologischen Entwicklung des Landes, der alternden Bevölkerung und des hohen Stellenwerts qualitativ hochwertiger Daten in klinischen Studien an Dynamik. Die japanische Regulierungsbehörde PMDA ist zunehmend offen für digitale Endpunkte und Tools zur Ferndatenerfassung. Der Markt wird zudem durch die Zunahme von klinischen Studien zu Hause und ambulant geprägt, was den Bedarf an präzisen und patientenfreundlichen eCOA-Systemen erhöht. Die Integration in breitere eClinical-Ökosysteme und KI-gestützte Patienteneinbindungstools dürften das Wachstum weiter beschleunigen.
Markteinblick in die elektronische klinische Ergebnisbewertung (eCOA) in Indien
Der indische Markt für elektronische klinische Ergebnisbewertungen (eCOA) erzielte 2024 den größten Umsatzanteil im asiatisch-pazifischen Raum. Dies ist auf einen Anstieg der klinischen Studien, eine technikaffine Bevölkerung und wachsende pharmazeutische Produktionskapazitäten zurückzuführen. Indiens kosteneffiziente CRO-Landschaft und die unterstützende Regierungspolitik zur Digitalisierung des Gesundheitswesens ermutigen globale Sponsoren, eCOA-Tools in nationalen Studien einzusetzen. Die zunehmende Verbreitung von Smartphones, das Wachstum der Telemedizin und die verbesserte Internetverbindung in städtischen und halbstädtischen Gebieten machen mobile und Cloud-basierte eCOA-Plattformen zugänglicher und weit verbreiteter.
Marktanteil der elektronischen klinischen Ergebnisbewertung (eCOA)
Die Branche der elektronischen klinischen Ergebnisbewertung (eCOA) wird hauptsächlich von etablierten Unternehmen angeführt, darunter:
- IQVIA (USA)
- Clario (USA)
- Medidata (USA)
- Veeva Systems (USA)
- Erdressourcentechnologie (USA)
- Oracle Health Sciences (USA)
- YPrime, LLC (USA)
- ArisGlobal LLC (USA)
- Castor EDC (Niederlande)
- eClinicalWorks (USA)
- Medrio, Inc. (USA)
- ClinOne (USA)
- Signant Health (USA)
- Clinical Ink, Inc. (USA)
- Curebase, Inc. (USA)
- Kayentis (Frankreich)
- Calyx (Großbritannien)
- Datacubed Health (USA)
- HealthDiary, Inc. (USA)
Neueste Entwicklungen auf dem globalen Markt für elektronische klinische Ergebnisbewertung (eCOA)
- Im Mai 2025 übernahm Clario (USA) das eCOA-Geschäft von WCG Clinical (USA). Dies ist ein strategischer Schritt, um seine Führungsposition bei digitalen Endpunktdatenlösungen, insbesondere für neurowissenschaftliche klinische Studien, zu stärken. Diese Übernahme erweitert Clarios umfassende Endpunktdatenplattform, ermöglicht eine bessere Unterstützung komplexer Studienumgebungen und festigt seine Position in der sich schnell entwickelnden eCOA-Landschaft weiter.
- Im Mai 2025 setzte das Critical Path Institute (USA) seine Initiative „eCOA: Gemeinsam besser werden“ fort, um Sponsoren, Technologieanbieter und Regulierungsbehörden zu vereinen. Diese bis März 2025 andauernde Zusammenarbeit konzentriert sich auf die Etablierung vorwettbewerblicher Best Practices und eines gemeinsamen Lexikons für die eCOA-Datenerfassung, die Förderung der Standardisierung und die Beschleunigung der Einführung in verschiedenen Regionen.
- Im November 2023 erweiterte Clinical Ink sein Patientenengagement-Paket um das Verhaltensdiagnosetool SPUR von Observia. Diese Integration kombiniert Verhaltensanalyse mit Lebensstilmodifikation, eCOA, eSource und digitalen Biomarkern und zielt darauf ab, ein ganzheitlicheres Verständnis des Patientenverhaltens zu ermöglichen und die Studienergebnisse zu verbessern.
- Im Oktober 2023 ging Clario eine strategische Partnerschaft mit Trial Data ein, einem Anbieter für dezentrale klinische Studien (DCT). Diese Zusammenarbeit stärkt Clarios Präsenz im chinesischen Bereich klinischer Studien und bündelt die Expertise der beiden Unternehmen, um hochmoderne dezentrale Studienlösungen zu liefern und patientenzentrierte Ansätze in der Region voranzutreiben.
- Im Dezember 2022 stellte Suvoda LLC, ein Unternehmen für eCOA-Technologie für klinische Studien, sein Design-Toolkit für elektronische klinische Ergebnisbewertungen (eCOA) vor. Dieses Toolkit ist für die nahtlose Integration mit Suvoda IRT und eConsent konzipiert, behebt historische Unzulänglichkeiten bei der eCOA-Implementierung und zielt darauf ab, den Designprozess zu optimieren.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.