Global Clinical Trial Imaging Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1.23 Billion
USD
2.29 Billion
2024
2032
USD
1.23 Billion
USD
2.29 Billion
2024
2032
| 2025 –2032 | |
| USD 1.23 Billion | |
| USD 2.29 Billion | |
|
|
|
|
Globale Marktsegmentierung für Bildgebung bei klinischen Studien nach Produkten und Dienstleistungen (Dienstleistungen und Software), Modalität (Computertomographie, Magnetresonanztomographie, Echokardiographie, Nuklearmedizin, Positronen-Emissions-Tomographie, Röntgen, Ultraschall, optische Kohärenztomographie und andere), Anwendung (Onkologie, Neurologie, Endokrinologie, Kardiologie, Dermatologie, Hämatologie und andere), Endbenutzer (Pharma- und Biotechnologieunternehmen, Auftragsforschungsinstitute, Hersteller medizinischer Geräte, akademische und staatliche Forschungsinstitute und andere), Distributor (Direktvertrieb und Ausschreibungsverkäufe) – Branchentrends und Prognose bis 2032.
Marktgröße für Bildgebung in klinischen Studien
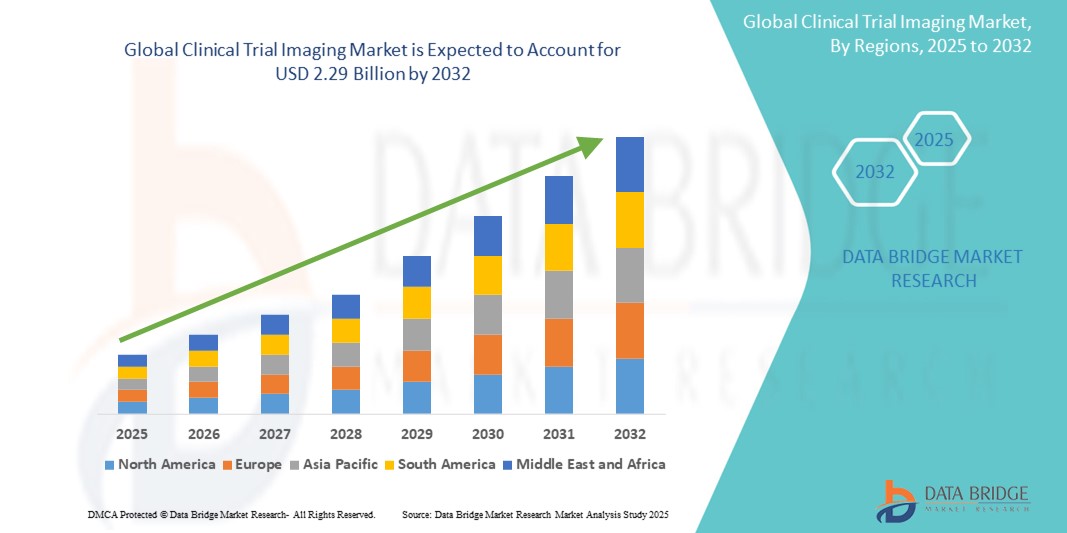
- Der globale Markt für Bildgebung bei klinischen Studien wurde im Jahr 2024 auf 1,23 Milliarden US-Dollar geschätzt und dürfte bis 2032 2,29 Milliarden US-Dollar erreichen.
- Im Prognosezeitraum von 2025 bis 2032 dürfte der Markt mit einer jährlichen Wachstumsrate von 8,1 % wachsen , was vor allem auf die steigende Nachfrage nach fortschrittlichen Bildgebungstechnologien und die steigende Zahl klinischer Studien weltweit zurückzuführen ist.
- Dieses Wachstum wird durch Faktoren wie den steigenden Bedarf an präzisen und nicht-invasiven Bildgebungslösungen zur Überwachung der Patientenreaktionen, technologische Fortschritte bei den Bildgebungsverfahren und die Expansion des Gesundheitssektors, insbesondere in den Schwellenländern, vorangetrieben.
Marktanalyse für Bildgebung in klinischen Studien
- Der Markt für die Bildgebung klinischer Studien wächst rasant, angetrieben durch den zunehmenden Einsatz fortschrittlicher Bildgebungstechnologien wie Magnetresonanztomographie und Positronen-Emissions-Tomographie in der Arzneimittelentwicklung und bei klinischen Studien.
- So hat beispielsweise der Einsatz von MRT in neurologischen Studien den Forschern geholfen, die Auswirkungen von Behandlungen auf Gehirnerkrankungen besser zu verstehen.
- Bildgebungstechnologien sind heute unverzichtbar für die Verfolgung des Krankheitsverlaufs und der Behandlungsreaktionen und helfen Forschern, die Auswirkungen neuer Therapien präziser zu überwachen.
- Beispielsweise werden CT-Scans in Herz-Kreislauf-Studien häufig verwendet, um die Herzfunktion und den Zustand der Arterien als Reaktion auf neue Medikamente zu beurteilen.
- Echtzeit-Bildgebungslösungen sind in onkologischen Studien unverzichtbar geworden, da dort eine genaue Tumorbeurteilung erforderlich ist, um die Wirksamkeit der Behandlung zu beurteilen, wie beispielsweise bei PET-Scans in der Krebsforschung.
- Beispielsweise werden PET-Scans häufig in Studien wie der Entwicklung gezielter Therapien für Lungenkrebs eingesetzt.
- Die Zusammenarbeit zwischen Bildgebungsunternehmen und Auftragsforschungsinstituten hat die Serviceintegration verbessert, die Arbeitsabläufe effizienter gestaltet und die Zeitabläufe bei klinischen Studien verkürzt. Unternehmen wie Covance und Parexel haben sich mit Bildgebungsfirmen zusammengeschlossen, um nahtlose Studienmanagementdienste anzubieten, die die Erfassung und Analyse von Bilddaten umfassen.
- Die Integration künstlicher Intelligenz in Bildgebungssysteme hat die Genauigkeit der Datenanalyse erhöht. KI hilft dabei, Veränderungen in Scans automatisch zu erkennen, den Beurteilungsprozess zu beschleunigen und menschliche Fehler zu reduzieren. KI ist besonders nützlich in klinischen Studien, beispielsweise zur Alzheimer-Krankheit, wo sie hilft, frühe Anzeichen einer Neurodegeneration in MRT-Scans zu erkennen.
Berichtsumfang und Marktsegmentierung für Bildgebung in klinischen Studien
|
Eigenschaften |
Wichtige Markteinblicke in die Bildgebung klinischer Studien |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen. |
Markttrends für die Bildgebung klinischer Studien
„ Zunehmende Nutzung fortschrittlicher Bildgebungstechnologien “
- Die zunehmende Nutzung fortschrittlicher Bildgebungstechnologien revolutioniert klinische Studien, indem sie hochauflösende, detaillierte Bilder liefern, die die Genauigkeit der gesammelten Daten verbessern
- So werden PET-Scans beispielsweise in onkologischen Studien heute routinemäßig eingesetzt, um die Reaktion des Tumors auf die Behandlung zu beurteilen und so eine präzisere Überwachung der Krebstherapien zu ermöglichen.
- Die Magnetresonanztomographie wird häufig in neurologischen Studien eingesetzt und ermöglicht es Forschern, Veränderungen in der Struktur und Funktion des Gehirns zu verfolgen.
- Beispielsweise ist die MRT in Studien zur Alzheimer-Krankheit von entscheidender Bedeutung, um das Fortschreiten der Neurodegeneration zu überwachen und die Auswirkungen potenzieller Behandlungen zu bewerten
- In der Herz-Kreislauf-Forschung werden fortschrittliche bildgebende Verfahren wie die Computertomographie eingesetzt, um die Herzfunktion und die Gefäßgesundheit zu beurteilen. Dadurch können Forscher die Auswirkungen neuer Medikamente auf das Herz-Kreislauf-System besser verstehen.
- Der Einsatz moderner Bildgebung in klinischen Studien der frühen Phase nimmt zu. Forscher können dadurch Arzneimittelwirkungen bereits in den frühesten Behandlungsphasen erkennen und messen. Dieser Trend ist besonders wertvoll für die Entwicklung neuer Therapien für Krankheiten wie Diabetes und chronische Schmerzen.
- Die Integration von Bildgebungstechnologien mit Echtzeit-Datenanalyse wird immer häufiger eingesetzt. Sie ermöglicht sofortiges Feedback zu Behandlungseffekten und hilft Klinikern, bei klinischen Studien schnellere und fundiertere Entscheidungen zu treffen.
Marktdynamik für Bildgebung in klinischen Studien
Treiber
„Steigende Nachfrage nach Präzisionsmedizin in klinischen Studien“
- Die wachsende Nachfrage nach Präzisionsmedizin ist einer der Haupttreiber des Marktes für bildgebende Verfahren für klinische Studien, da die Behandlungen zunehmend auf die einzelnen Patienten zugeschnitten werden, basierend auf ihrer genetischen Ausstattung, ihrem Lebensstil und anderen Faktoren.
- In klinischen Studien in der Onkologie werden fortschrittliche Bildgebungsverfahren wie Magnetresonanztomographie und Positronen-Emissions-Tomographie eingesetzt, um die Tumorgröße zu überwachen, Metastasen zu erkennen und zu bewerten, wie Patienten auf bestimmte Behandlungen wie zielgerichtete Therapien oder Immuntherapien reagieren.
- Durch die Echtzeitüberwachung mittels Bildgebung können Forscher Behandlungspläne an die individuelle Reaktion des Patienten anpassen und so die Gesamtwirksamkeit personalisierter Behandlungen verbessern.
- Beispielsweise werden PET-Scans verwendet, um zu verfolgen, wie gut der Tumor eines Krebspatienten schrumpft, und helfen Ärzten bei der Entscheidung, ob der Behandlungsplan fortgesetzt oder geändert werden soll.
- Auch in neurologischen Studien gewinnt die Präzisionsmedizin zunehmend an Bedeutung. Bildgebungsverfahren wie die Magnetresonanztomographie (MRT) dienen hier der Überwachung des Krankheitsverlaufs bei Erkrankungen wie Alzheimer. So können Medikamentenprotokolle an die individuellen Reaktionen angepasst werden.
- Da Pharmaunternehmen weiterhin in personalisierte Therapien investieren, wird der Bedarf an fortschrittlichen Bildgebungslösungen zur Beurteilung der Wirksamkeit dieser Therapien weiter steigen und die Innovation im Markt für Bildgebung bei klinischen Studien weiter vorantreiben.
Gelegenheit
„Integration künstlicher Intelligenz in die Bildgebung“
- Die Integration künstlicher Intelligenz in die Bildgebung bei klinischen Studien bietet große Wachstumschancen, da sie die Art und Weise verändert, wie klinische Daten erfasst, analysiert und interpretiert werden.
- Beispielsweise werden KI-gestützte Bildgebungsplattformen, wie sie von Zebra Medical Vision entwickelt wurden, zur Analyse großer Mengen medizinischer Bilder eingesetzt, wodurch der Zeitaufwand für Diagnose und Behandlungsplanung erheblich reduziert wird.
- KI-gestützte Bildgebungslösungen sind besonders in onkologischen, neurologischen und kardiologischen Studien von Nutzen, da hier eine frühzeitige Erkennung und präzise Überwachung des Krankheitsverlaufs unerlässlich sind. In Krebsstudien werden KI-Algorithmen eingesetzt, um Tumorwachstum oder -schrumpfung automatisch zu erkennen. Dies zeigt sich beispielsweise in Studien mit KI-gesteuerten Systemen, die PET-Scans analysieren, um die Behandlung von Lungenkrebs genauer zu überwachen.
- KI-Algorithmen können auch subtile Veränderungen in Bilddaten erkennen, wie etwa frühe Anzeichen eines Krankheitsverlaufs oder Nebenwirkungen, die für menschliche Beobachter möglicherweise nicht sofort sichtbar sind. Dies verbessert die Patientensicherheit in klinischen Studien. In Alzheimer-Studien wird KI eingesetzt, um frühe Gehirnveränderungen in MRT-Scans zu erkennen und so die Wirksamkeit von Medikamenten zu ermitteln, bevor Symptome auftreten.
- Die kontinuierliche Lernfähigkeit von KI-Algorithmen ermöglicht es ihnen, ihre Genauigkeit im Laufe der Zeit zu verbessern und präzisere Vorhersagen und Erkenntnisse zu ermöglichen, wenn sie mit größeren Datensätzen konfrontiert werden.
- Beispielsweise wurde IBMs Watson Health eingesetzt, um die Analyse radiologischer Bilder zu verbessern und so die diagnostische Genauigkeit in klinischen Studien zu Herz-Kreislauf-Erkrankungen im Laufe der Zeit zu verbessern.
- Durch die Integration von KI in die Bildgebung klinischer Studien können Pharmaunternehmen und Forschungseinrichtungen Arbeitsabläufe optimieren, menschliche Fehler reduzieren und den klinischen Studienprozess beschleunigen. Dies eröffnet dem Markt in den kommenden Jahren erhebliche Wachstumschancen. KI-Systeme wie die Radiologie-Software von Aidoc werden bereits von klinischen Prüforganisationen eingesetzt, um die Effizienz und die Geschwindigkeit der Datenverarbeitung während der Studien zu verbessern.
Einschränkung/Herausforderung
„Hohe Kosten für fortschrittliche Bildgebungstechnologien“
- Eine der größten Herausforderungen für den Markt für Bildgebung bei klinischen Studien sind die hohen Kosten, die mit fortschrittlichen Bildgebungstechnologien verbunden sind.
- Beispielsweise erfordern Techniken wie Magnetresonanztomographie, Positronenemissionstomographie und Computertomographie erhebliche Investitionen sowohl in die Ausrüstung als auch in deren laufende Wartung, was für kleinere Forschungsorganisationen eine finanzielle Belastung darstellen kann.
- Die Installation und Wartung moderner Bildgebungssysteme kann unerschwinglich teuer sein, insbesondere in Schwellenländern, wo die Budgets für klinische Studien begrenzter sind. Dies kann die Teilnahme kleinerer Forschungseinrichtungen oder Krankenhäuser an anspruchsvollen klinischen Studien einschränken und den Zugang zu wichtigen Bildgebungstechnologien einschränken.
- Neben den Anschaffungskosten erfordert die Komplexität des Betriebs dieser Technologien auch hochqualifiziertes Personal
- Beispielsweise werden in onkologischen Studien spezialisierte Radiologen benötigt, um PET-Scan-Bilder zu interpretieren. Der Bedarf an Expertenanalysen erhöht sowohl den Zeit- als auch den Kostenaufwand der Studie und trägt zu den Gesamtkosten bei.
- Die finanzielle Belastung durch teure Bildgebungstechnologien kann zu Budgeteinschränkungen für Sponsoren klinischer Studien führen, insbesondere bei groß angelegten Studien mit vielfältigen Bildgebungsanforderungen. Dies kann die Anzahl der durchgeführten Studien begrenzen oder die Einführung neuerer Bildgebungsverfahren verzögern. Ein Beispiel hierfür ist der Einsatz KI-gestützter PET-Scans, die zwar die Effizienz steigern, aber dennoch erhebliche Investitionen erfordern.
- In ressourcenbeschränkten Umgebungen stellen die hohen Kosten für fortschrittliche Bildgebungstechnologien ein erhebliches Hindernis dar
- Beispielsweise ist es bei klinischen Studien in Entwicklungsländern oft schwierig, hochauflösende Bildgebungstechnologien zu integrieren, da die finanziellen Mittel sowohl für die Ausrüstung als auch für ausgebildetes Fachpersonal begrenzt sind. Für diese Regionen ist es daher eine Herausforderung, Zugang zu hochmodernen Bildgebungslösungen zu erhalten.
Marktumfang für Bildgebung bei klinischen Studien
Der Markt ist nach Produkt und Dienstleistung, Modalität, Anwendung, Endbenutzer und Distributor segmentiert
|
Segmentierung |
Untersegmentierung |
|
Nach Produkten und Dienstleistungen |
|
|
Nach Modalität |
|
|
Nach Anwendung |
|
|
Nach Endbenutzer |
|
|
Nach Distributor |
|
Regionale Analyse des Marktes für Bildgebung in klinischen Studien
„Nordamerika ist die dominierende Region auf dem Markt für Bildgebung bei klinischen Studien“
- Nordamerika dominiert den Markt für Bildgebung bei klinischen Studien, vor allem aufgrund der günstigen Erstattungspolitik der USA, die erhebliche Investitionen in Forschung und Entwicklung, insbesondere in Bildgebungstechnologien, fördert.
- Diese Erstattungsrichtlinien haben zu kontinuierlichen Fortschritten bei Bildgebungsverfahren wie Magnetresonanztomographie, Positronen-Emissions-Tomographie und Computertomographie geführt und stellen sicher, dass Nordamerika bei Innovationen im Bereich klinischer Studien weiterhin eine Vorreiterrolle einnimmt.
- Der Aufstieg der Auftragsforschungsinstitute (CROs) in den USA und Kanada hat zur Dominanz der Region beigetragen, indem sie spezialisierte Dienstleistungen wie die Verwaltung von Bilddaten und die Anwendung fortschrittlicher Technologien zur besseren Überwachung von Patienten und Krankheitsverlauf anbieten.
- CROs spielen eine entscheidende Rolle bei der Verbesserung der Effizienz und Qualität klinischer Studien, was die Führungsrolle Nordamerikas in der Bildgebung klinischer Studien weiter stärkt.
- Dank einer starken Gesundheitsinfrastruktur, einer hohen Anzahl klinischer Studien und einem zunehmenden Fokus auf Präzisionsmedizin und KI-gesteuerte Bildgebungslösungen wächst Nordamerika weiter und behauptet seine beherrschende Stellung auf dem Markt für klinische Studienbildgebung.
„Asien-Pazifik wird voraussichtlich die höchste Wachstumsrate verzeichnen“
- Der asiatisch-pazifische Raum ist der am schnellsten wachsende Markt für die Bildgebung bei klinischen Studien. Angetrieben wird er von Ländern wie China, Indien und Japan , wo Fortschritte in der Gesundheitsinfrastruktur, regulatorische Reformen und erhöhte Investitionen in die klinische Forschung rasch voranschreiten.
- China hat sich zu einem Zentrum für klinische Studien entwickelt und bietet eine große Patientenpopulation und optimierte Regulierungsprozesse, die das Land zu einem attraktiven Standort für Pharmaunternehmen machen, die sich in der frühen Phase der Arzneimittelentwicklung befinden.
- Indien trägt mit seinen kostengünstigen Lösungen für klinische Studien und seinem vielfältigen Patientenpool erheblich zum Wachstum bei und bietet globalen Pharmaunternehmen die Möglichkeit, Kosten zu minimieren und gleichzeitig die Arzneimittelentwicklung in verschiedenen Therapiebereichen zu beschleunigen.
- Japan investiert massiv in Präzisionsmedizin und fortschrittliche Bildgebungstechnologien, insbesondere für onkologische und neurologische Studien. Der Fokus des Landes auf Spitzentechnologien verbessert die Gesamtqualität klinischer Studien in der Region.
- Die steigende Zahl von Auftragsforschungsinstituten (CROs) und die verstärkte Zusammenarbeit zwischen Technologieunternehmen und Forschungseinrichtungen im asiatisch-pazifischen Raum beschleunigen das Wachstum des Marktes weiter und machen ihn in den kommenden Jahren zu einem führenden Anbieter im Bereich der Bildgebung für klinische Studien.
Marktanteil der Bildgebung für klinische Studien
Die Wettbewerbslandschaft des Marktes liefert detaillierte Informationen zu den einzelnen Wettbewerbern. Zu den Details gehören Unternehmensübersicht, Unternehmensfinanzen, Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben genannten Datenpunkte beziehen sich ausschließlich auf die Marktausrichtung der Unternehmen.
Die wichtigsten Marktführer auf dem Markt sind:
- Navitas Life Sciences (USA)
- Resonance Health Ltd. (Australien)
- BioTelemetry, ein Philips-Unternehmen (USA)
- IXICO plc (Großbritannien)
- ICON plc (Irland)
- Image Core Lab (Indien)
- Anagramm 4 klinische Studien (Spanien)
- Quotient Sciences (Großbritannien)
- Radiant Sage LLC (USA)
- WORLDCARE CLINICAL (USA)
- Clario (USA)
- Parexel International Corporation (USA)
- Median Technologies (Frankreich)
- Perspectum (Großbritannien)
- Kelch (Nottingham)
- Invicro LLC (USA)
Neueste Entwicklungen auf dem globalen Markt für Bildgebung in klinischen Studien
- Im Mai 2022 brachte Bruker neuartige präklinische 7-Tesla- und 9,4-Tesla-MRT-Magnete auf den Markt , die die Präzision der Bildgebung in der klinischen Forschung verbessern sollen. Diese fortschrittlichen MRT-Systeme bieten eine höhere Auflösung und ermöglichen Forschern detailliertere Einblicke in Krankheitsmechanismen auf molekularer Ebene. Die neue Technologie soll die Effizienz und Genauigkeit präklinischer Studien, insbesondere in der Onkologie, Neurologie und Herz-Kreislauf-Forschung, verbessern. Diese Entwicklung wird den Markt beeinflussen, indem sie die Bildgebungsmöglichkeiten verbessert, die Arzneimittelentwicklung beschleunigt und präzisere Bewertungen der therapeutischen Wirksamkeit ermöglicht.
- Im März 2022 stellte Fujifilm India auf der 74. IRIA-Konferenz (Indian Radiological and Imaging Association) eine neue Produktreihe vor. Das Unternehmen präsentierte fortschrittliche Bildgebungslösungen, darunter diagnostische Bildgebungstechnologien zur Verbesserung der Gesundheitsversorgung in Indien. Diese Produkte sollen die Qualität der medizinischen Bildgebung verbessern und medizinisches Fachpersonal bei der Erstellung präziserer Diagnosen unterstützen. Die Einführung dieser Technologien wird den Markt erheblich beeinflussen, indem sie den Zugang zu hochwertigen Bildgebungslösungen erweitert, die Leistungsfähigkeit medizinischer Einrichtungen verbessert und den Fortschritt klinischer Studien beschleunigt.
- Im Januar 2022 fusionierten ERT und BioClinica zu Clario , einem führenden Technologieunternehmen für Innovationen im Bereich klinischer Studien. Die Fusion vereint die Expertise von ERT in der Datenerfassung klinischer Studien mit den Bildgebungslösungen von BioClinica und schafft so eine einheitliche Plattform zur Steigerung der Studieneffizienz. Clario zielt darauf ab, die Prozesse klinischer Studien zu optimieren und integrierte Dienstleistungen für Datenerfassung und Bildgebung anzubieten, um Genauigkeit, Geschwindigkeit und Patientenergebnisse zu verbessern. Es wird erwartet, dass diese Entwicklung den Markt maßgeblich beeinflussen wird, indem sie die Technologie klinischer Studien vorantreibt und Innovationen vorantreibt.
- Im November 2021 erweiterten Clario und XingImaging ihre Partnerschaft, um PET-Bildgebungslösungen für klinische Studien in China anzubieten. Diese Entwicklung zielt darauf ab, die Genauigkeit und Effizienz klinischer Studien durch die Integration fortschrittlicher PET-Bildgebungstechnologie mit Clarios Datenerfassungs- und Analysefunktionen zu verbessern. Die Partnerschaft ermöglicht Pharmaunternehmen und CROs die Durchführung präziserer Studien und verbessert so die Überwachung des Krankheitsverlaufs und der Behandlungswirksamkeit. Diese Erweiterung wird dem Markt erhebliche Vorteile bringen, indem sie Zugang zu hochwertigen Bildgebungsdiensten in China bietet und die Arzneimittelentwicklung in der Region beschleunigt.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.