Global Biosimilar Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
64.93 Billion
USD
598.55 Billion
2024
2032
USD
64.93 Billion
USD
598.55 Billion
2024
2032
| 2025 –2032 | |
| USD 64.93 Billion | |
| USD 598.55 Billion | |
|
|
|
|
Globale Biosimilar-Marktsegmentierung nach Produkttyp (Magnetresonanztomographie-Scanner, Computertomographie-Scanner, Positronenemissionstomographie-Scanner, Biosimilar (EEG), Elektromyographie-Geräte (EMG), Magnetoenzephalographie-Geräte, transkranielle Doppler-Geräte, intrakranielle Druckmonitore (ICP), Elektroden, Sensoren und Gele und Kabel), Medikamentenklasse (Insulin, rekombinantes menschliches Wachstumshormon (RHGH), Granulozyten-Kolonie-stimulierender Faktor, Interferon, Erythropoietin, Etanercept, monoklonale Antikörper, Follitropin, Glucagon, Calcitonin, Teriparatid und Enoxaparin-Natrium), Herstellungsart (Eigenherstellung und Auftragsfertigung), Verfahren (invasiv und nicht-invasiv), Krankheit (Schlaganfall, Demenz und Epilepsie), Indikation (Vorhofseptumdefekt (ASD), Ventrikelseptumdefekt (VSD), persistierendes Foramen ovale (PFO), Aortenklappenstenose und andere), Therapietyp (Onkologie, Immunologie, Hämatologie, Hormontherapie, Stoffwechselstörungen und andere), Endbenutzer (Krankenhäuser, Kliniken, Diagnosezentren und andere) – Branchentrends und Prognose bis 2032
Biosimilar-Marktgröße
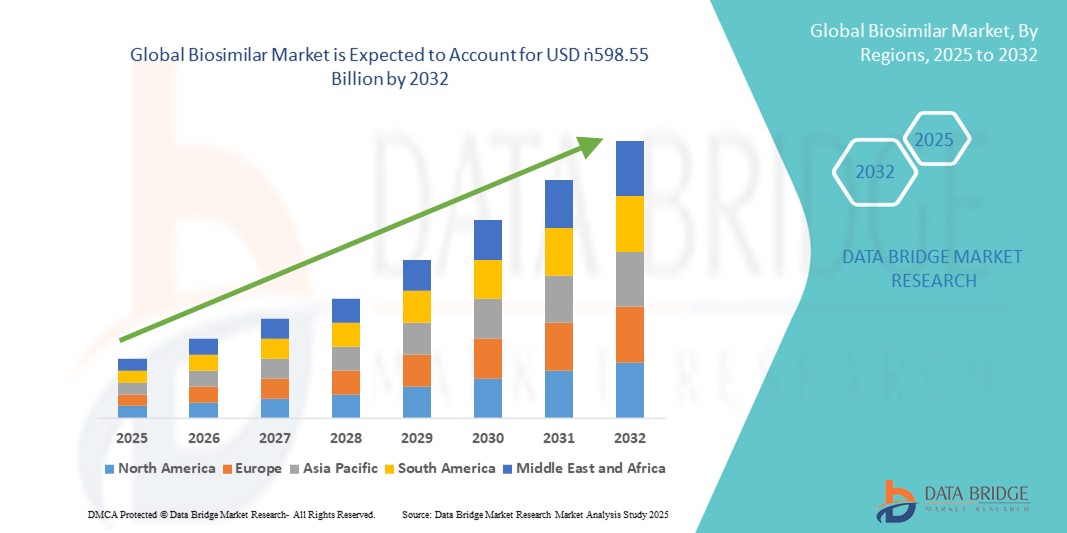
- Der globale Biosimilar-Markt wird im Jahr 2024 auf 64,93 Milliarden US-Dollar geschätzt und soll bis 2032 598,55 Milliarden US-Dollar erreichen , bei einer CAGR von 32,00 % im Prognosezeitraum.
- Die Marktexpansion ist vor allem auf die steigende Zahl von Patentabläufen bei Blockbuster-Biologika und die zunehmende Verbreitung chronischer Krankheiten wie Krebs, Autoimmunerkrankungen und Diabetes zurückzuführen, die die Nachfrage nach kostengünstigen Alternativen ankurbeln.
- Darüber hinaus beschleunigen günstige regulatorische Rahmenbedingungen, steigende Investitionen von Pharmaunternehmen und eine größere Akzeptanz bei Gesundheitsdienstleistern die Entwicklung und Einführung von Biosimilars weltweit. Diese Trends tragen maßgeblich zum starken Wachstum der Biosimilar-Industrie bei.
Biosimilar-Marktanalyse
- Biosimilars, die als sehr ähnliche und kostengünstige Alternativen zu zugelassenen biologischen Arzneimitteln konzipiert sind, werden zu einem entscheidenden Bestandteil globaler Gesundheitssysteme, da sie den Zugang zu Behandlungen verbessern, die Gesundheitskosten senken und den Patientenkreis bei chronischen und lebensbedrohlichen Erkrankungen wie Krebs, Autoimmunerkrankungen und Diabetes erweitern.
- Die steigende Nachfrage nach Biosimilars wird vor allem durch die Welle von Patentabläufen bei Blockbuster-Biologika, den zunehmenden Druck auf die Gesundheitsausgaben und die zunehmende Akzeptanz von Biosimilars bei Anbietern und Patienten als sichere und wirksame Therapieoptionen angeheizt.
- Nordamerika dominierte den globalen Biosimilar-Markt mit dem größten Umsatzanteil von 42,8 % im Jahr 2024. Dies ist auf die starke regulatorische Unterstützung der US-amerikanischen FDA, den zunehmenden Einsatz von Biosimilars in hochwertigen Therapiekategorien und die zunehmende Akzeptanz bei Kostenträgern zurückzuführen, die nach erschwinglichen Alternativen zu teuren Biologika suchen. Insbesondere die USA verzeichneten aufgrund günstiger politischer Veränderungen, wettbewerbsfähiger Preise und der Präsenz führender Pharmaunternehmen eine schnelle Marktdurchdringung.
- Der asiatisch-pazifische Raum dürfte im Prognosezeitraum die am schnellsten wachsende Region im Biosimilar-Markt sein, aufgrund der wachsenden Gesundheitsinfrastruktur, der steigenden Zahl chronischer Krankheiten und unterstützender Regierungsinitiativen in Ländern wie China, Indien und Südkorea.
- Das Onkologiesegment dominierte den Biosimilar-Markt mit einem Umsatzanteil von 42,2 % im Jahr 2024, bedingt durch die hohen Kosten für Referenzbiologika, die weltweit zunehmende Verbreitung von Krebs und die schnelle Akzeptanz von biosimilaren monoklonalen Antikörpern und unterstützenden Therapien.
Berichtsumfang und Biosimilar-Marktsegmentierung
|
Eigenschaften |
Wichtige Markteinblicke zu Biosimilars |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Nordamerika
Europa
Asien-Pazifik
Naher Osten und Afrika
Südamerika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Biosimilar-Markttrends
Ausbau der Zugänglichkeit durch günstige Regelungen und Kosteneinsparungen
- Ein bedeutender und sich beschleunigender Trend auf dem globalen Biosimilar-Markt ist die Ausweitung regulatorischer Unterstützung und politischer Rahmenbedingungen, die eine schnellere Zulassung und eine breitere Akzeptanz von Biosimilars als kostengünstige Alternative zu teuren Biologika fördern. Dies verändert die Erschwinglichkeit von Behandlungen und die Patientenzugänglichkeit in zahlreichen Therapiebereichen.
- So veröffentlichte die US-amerikanische FDA im Jahr 2023 neue Leitlinien zur Austauschbarkeit, um den Markteintritt von Biosimilars zu vereinfachen, während die Europäische Arzneimittelagentur (EMA) weiterhin die weltweit höchste Anzahl an Biosimilar-Zulassungen verzeichnete. Diese Maßnahmen stärken das Vertrauen der Branche und fördern den wettbewerbsfähigen Markteintritt.
- Der zunehmende Kostendruck im Gesundheitswesen zwingt Kostenträger und Regierungen dazu, der Einführung von Biosimilars Priorität einzuräumen. In den USA beispielsweise kam Amgens Amjevita, ein Biosimilar zu Humira, 2023 zu deutlich niedrigeren Kosten auf den Markt als AbbVies Referenzbiologikum und schuf damit einen Präzedenzfall für eine verstärkte Markteinführung. Auch Biocon und Viatris führen im asiatisch-pazifischen Raum aktiv Biosimilars für Onkologie und Diabetes zu erschwinglichen Preisen ein.
- Dieser Trend wird durch das wachsende Vertrauen der Ärzte verstärkt, da immer mehr Praxisnachweise bestätigen, dass Biosimilars eine mit Originalbiologika vergleichbare Sicherheit und Wirksamkeit bieten. Umfangreiche Aufklärungskampagnen für Ärzte und Patienten beschleunigen die Akzeptanz in Therapiebereichen wie Onkologie, Autoimmunerkrankungen und Diabetes zusätzlich.
- Darüber hinaus ermöglichen Partnerschaften zwischen globalen Pharmaunternehmen und regionalen Akteuren eine kosteneffiziente Entwicklung und Distribution. So hat Samsung Bioepis beispielsweise seine Reichweite durch Kooperationen mit Organon und Biogen erweitert, um sein Biosimilar-Portfolio in den Bereichen Immunologie und Ophthalmologie zu stärken.
- Dieser regulatorische und wirtschaftliche Druck zur Einführung von Biosimilars verändert die globalen Biologika-Märkte grundlegend. Die Nachfrage steigt sowohl in Industrie- als auch in Schwellenländern rasant, da die Interessengruppen zunehmend Wert auf Kosteneinsparungen und die Zugänglichkeit der Behandlung legen.
Marktdynamik für Biosimilars
Treiber
Steigende Nachfrage durch Patentablauf bei Biologika und chronische Krankheitslast
- Der Anstieg der Nachfrage nach Biosimilars ist vor allem auf den Verlust der Patentexklusivität für Blockbuster-Biologika wie Humira, Herceptin und Avastin zurückzuführen. Dadurch ergeben sich Möglichkeiten für kostengünstigere Biosimilars, bedeutende Marktanteile zu erobern.
- So werden beispielsweise im Jahr 2023 in den USA mehrere Humira-Biosimilars von Unternehmen wie Amgen, Boehringer Ingelheim und Sandoz auf den Markt kommen, was zu einem wettbewerbsfähigen Preisumfeld und einem breiteren Patientenzugang führen wird.
- Die weltweit wachsende Belastung durch chronische Krankheiten wie Krebs, Autoimmunerkrankungen und Diabetes verstärkt den Bedarf an erschwinglichen biologischen Therapien und macht Biosimilars zu einem entscheidenden Faktor für die Nachhaltigkeit des Gesundheitssystems.
- Regierungen und Versicherer setzen zunehmend Erstattungsanreize und Substitutionsrichtlinien ein, um die Akzeptanz von Biosimilars zu fördern und so ein günstiges Umfeld für Hersteller zu schaffen.
- Das wachsende Bewusstsein von Ärzten und Patienten hinsichtlich der Wirksamkeit und Sicherheit von Biosimilars im Vergleich zu Originalpräparaten fördert die Akzeptanz zusätzlich, unterstützt durch laufende Aufklärungsinitiativen der Gesundheitsbehörden.
Einschränkung/Herausforderung
Austauschbarkeitsbarrieren und Fertigungskomplexität
- Trotz guter Wachstumsaussichten behindern Herausforderungen im Zusammenhang mit der Austauschbarkeit und der regulatorischen Komplexität weiterhin eine breite Akzeptanz. In den USA bleibt die Bezeichnung der Austauschbarkeit eine kritische Hürde, da nicht alle Biosimilars auf Apothekenebene automatisch substituiert werden.
- So war beispielsweise Amgens Amjevita bei der Markteinführung in den USA zunächst nicht austauschbar, was die Substitution im Vergleich zu herkömmlichen Generika verlangsamte. Dies stellt für neue Marktteilnehmer, die schnell Fuß fassen wollen, weiterhin eine Herausforderung dar.
- Die Herstellung von Biosimilars ist zudem mit hohen technischen Hürden verbunden, da es sich bei Biologika um große, komplexe Moleküle handelt, die fortschrittliche Produktions- und Qualitätskontrollsysteme erfordern. Jede Abweichung im Produktionsprozess kann regulatorische Bedenken hervorrufen und die Zulassung verzögern.
- Darüber hinaus besteht in bestimmten Regionen weiterhin Zurückhaltung bei Ärzten und Patienten, da weiterhin Missverständnisse hinsichtlich der Wirksamkeit und Sicherheit von Biosimilars bestehen. Kontinuierliche Aufklärung und praxisnahe Daten sind unerlässlich, um diese Vorstellungen zu überwinden.
- Eine weitere Herausforderung stellt der Preisverfall aufgrund des harten Wettbewerbs dar, da Unternehmen unter dem Druck stehen, ihre Kosten zu senken und gleichzeitig hohe Entwicklungs- und Produktionskosten zu bewältigen. Dies kann die Rentabilität kleinerer Akteure, die in den Markt eintreten, einschränken.
- Die Überwindung dieser Hürden durch klarere regulatorische Wege, verbesserte Produktionskapazitäten und eine stärkere Aufklärung der Interessengruppen wird für ein nachhaltiges Wachstum im Biosimilar-Sektor von entscheidender Bedeutung sein.
Biosimilar-Marktumfang
Der Markt ist nach Produkttyp, Arzneimittelklasse, Herstellungsart, Verfahren, Krankheit, Indikation, Therapieart und Endverbrauchern segmentiert
- Nach Produkttyp
Der Biosimilar-Markt ist nach Produkttyp segmentiert in Magnetresonanztomographen, Computertomographen, Positronen-Emissions-Tomographen, Biosimilars (EEG), Elektromyographie-Geräte (EMG), Magnetoenzephalographie-Geräte, transkranielle Doppler-Geräte, Hirndruckmessgeräte (ICP), Elektroden, Sensoren, Gele und Kabel. Das Segment der Magnetresonanztomographen dominierte 2024 den Markt mit dem größten Umsatzanteil, getrieben von seiner entscheidenden Rolle in der Onkologie und Neurologie für hochauflösende Bildgebung und Krankheitsüberwachung. Die Nachfrage nach MRT wird zusätzlich durch ihre breite Anwendung bei der Erkennung von Tumoren, Gelenkerkrankungen und Herz-Kreislauf-Erkrankungen angeheizt, was mit der steigenden Prävalenz chronischer Krankheiten einhergeht. Krankenhäuser und Diagnosezentren bevorzugen MRT-basierte Biosimilars aufgrund ihrer Zuverlässigkeit, Sicherheit und verbesserten diagnostischen Genauigkeit. Darüber hinaus unterstützen günstige Erstattungsrichtlinien in entwickelten Märkten die stärkere Akzeptanz von MRT-Technologien im klinischen Umfeld.
Das Segment Elektroden wird voraussichtlich zwischen 2025 und 2032 das schnellste Wachstum verzeichnen, da sie in verschiedenen diagnostischen Verfahren weit verbreitet sind. Ihre Erschwinglichkeit, einfache Entsorgung und die steigende Nachfrage in der Neurologie und Kardiologie machen sie in Schwellenländern, in denen Kosteneffizienz entscheidend ist, äußerst attraktiv. Die rasante Urbanisierung und ein wachsender Patientenstamm, der EEG- und EMG-Überwachung benötigt, treiben die Akzeptanz zusätzlich voran. Darüber hinaus führt der Trend zu Point-of-Care-Diagnostik und tragbaren Überwachungsgeräten zu einer zunehmenden Nutzung dieser Verbrauchsmaterialien. Das Wachstum des Segments wird auch durch Fortschritte in der Materialwissenschaft unterstützt, die die Elektrodenempfindlichkeit und den Patientenkomfort verbessern.
- Nach Arzneimittelklasse
Auf der Grundlage der Arzneimittelklassen ist der Biosimilar-Markt in Insulin, rekombinantes humanes Wachstumshormon (RHGH), Granulozyten-Kolonie-stimulierender Faktor (G-CSF), Interferon, Erythropoietin, Etanercept, monoklonale Antikörper, Follitropin, Glucagon, Calcitonin, Teriparatid und Enoxaparin-Natrium segmentiert. Das Segment der monoklonalen Antikörper dominierte den Markt im Jahr 2024 aufgrund seiner entscheidenden Bedeutung in der Onkologie, Immunologie und bei Autoimmunerkrankungen. Der Ablauf der Patente für Blockbuster-Medikamente mit monoklonalen Antikörpern und die hohen Behandlungskosten haben einen starken Anreiz für die Entwicklung von Biosimilars geschaffen. Diese Biologika werden häufig zur Behandlung von Krebs, rheumatoider Arthritis und entzündlichen Darmerkrankungen eingesetzt und sind daher eine beliebte Wahl für Biosimilar-Hersteller. Das Segment profitiert außerdem von regulatorischen Verfahren, die schnellere Zulassungen und klinische Akzeptanz fördern.
Das Insulinsegment wird voraussichtlich zwischen 2025 und 2032 das schnellste Wachstum verzeichnen, angetrieben durch die weltweit steigende Prävalenz von Diabetes. Die wachsende globale Diabetikerpopulation, insbesondere im asiatisch-pazifischen Raum, führt zu einer starken Nachfrage nach erschwinglichen Insulinalternativen. Mehrere Insulin-Biosimilars haben die behördlichen Zulassungen erhalten, was die Marktdurchdringung beschleunigt. Regierungen und Gesundheitssysteme fördern aktiv Insulin-Biosimilars, um die Behandlungskosten zu senken und die Zugänglichkeit für Patienten zu verbessern. Darüber hinaus ermöglichen verbesserte Fertigungstechnologien und Partnerschaften zwischen Pharmaunternehmen eine höhere Verfügbarkeit.
- Nach Fertigungsart
Der Biosimilar-Markt wird nach Herstellungsart in Eigenproduktion und Auftragsproduktion unterteilt. Das Segment der Eigenproduktion dominierte den Markt im Jahr 2024, da große Pharmaunternehmen die Kontrolle über die Produktion behalten, um Qualität und Einhaltung strenger Biosimilar-Vorschriften sicherzustellen. Eigene Anlagen ermöglichen es Unternehmen, geistiges Eigentum zu verwalten, die Logistik der Lieferkette zu kontrollieren und die Produktionskosten langfristig zu optimieren. Sie gewährleisten zudem eine gleichbleibende Produktqualität, die entscheidend für das Vertrauen von Ärzten und Patienten in Biosimilars ist. Größere Pharmaunternehmen mit etablierter Biologika-Infrastruktur setzen weiterhin stark auf eigene Systeme, um sich Wettbewerbsvorteile zu sichern.
Das Segment Auftragsfertigung wird im Prognosezeitraum voraussichtlich am schnellsten wachsen, da mittelständische und kleine Biosimilar-Entwickler zunehmend Outsourcing betreiben. Auftragshersteller (CMOs) bieten spezialisiertes Know-how, Skalierbarkeit und Kosteneffizienz und ermöglichen so neuen Akteuren einen schnelleren Markteintritt. Die wachsende Pipeline an Biosimilars sowie steigende F&E-Aktivitäten in Schwellenländern steigern die Nachfrage nach Outsourcing-Partnerschaften. Darüber hinaus erweitern CMOs ihre Kapazitäten mit fortschrittlichen Bioreaktorsystemen und gesetzeskonformen Anlagen, um den Anforderungen globaler Kunden gerecht zu werden. Strategische Kooperationen zwischen Pharmaunternehmen und CMOs treiben das Wachstum dieses Segments weiter voran.
- Nach Verfahren
Der Markt für Biosimilars wird je nach Verfahren in invasiv und nicht-invasiv unterteilt. Das nicht-invasive Segment dominierte den Markt im Jahr 2024 mit dem größten Umsatzanteil, was auf die zunehmende Präferenz der Patienten für weniger schmerzhafte, sicherere und bequemere Therapieoptionen zurückzuführen ist. Nicht-invasive Ansätze werden häufig in der Onkologie und im Management chronischer Krankheiten eingesetzt, wo die Patienten-Compliance entscheidend ist. Auch Aufsichtsbehörden und Krankenhäuser bevorzugen nicht-invasive Behandlungen aufgrund des geringeren Komplikationsrisikos, der kürzeren Krankenhausaufenthalte und der niedrigeren Behandlungskosten. Der Aufstieg fortschrittlicher Bildgebung, diagnostischer Biosimilars und zielgerichteter Therapien festigt die Dominanz dieser Kategorie weiter.
Das invasive Segment wird voraussichtlich zwischen 2025 und 2032 die schnellste Wachstumsrate verzeichnen, angetrieben durch die steigende Nachfrage nach präzisen Eingriffen bei komplexen Erkrankungen wie Herz-Kreislauf- und neurologischen Störungen. Invasive Biosimilars sind für anspruchsvolle Operationen und zielgerichtete Therapien, bei denen die direkte Verabreichung von Biologika erforderlich ist, von entscheidender Bedeutung. Die steigende Zahl von Intensivpatienten, die chirurgische Eingriffe benötigen, treibt die Akzeptanz weiter voran. Darüber hinaus verbessern Fortschritte bei minimalinvasiven Operationstechniken die Sicherheitsprofile und führen zu einer breiteren Akzeptanz. Das Wachstum des Segments wird auch durch steigende Investitionen in die Gesundheitsinfrastruktur in Entwicklungsländern unterstützt.
- Durch Krankheit
Der Biosimilar-Markt ist nach Krankheitsbildern in die Segmente Schlaganfall, Demenz und Epilepsie unterteilt. Das Schlaganfallsegment dominierte den Markt im Jahr 2024 mit dem höchsten Umsatzanteil, vor allem aufgrund der weltweit zunehmenden Inzidenz ischämischer und hämorrhagischer Schlaganfälle. Biosimilars spielen eine entscheidende Rolle bei der Verbesserung der Erschwinglichkeit fortschrittlicher Therapien in der Schlaganfallnachsorge. Krankenhäuser und Rehabilitationszentren setzen zunehmend auf biosimilarbasierte Therapien, um die Genesung zu unterstützen und die finanzielle Belastung der Patienten zu reduzieren. Staatliche Initiativen zur Verbesserung des Zugangs zu erschwinglichen Schlaganfallbehandlungen stärken die Dominanz dieses Segments weiter.
Das Demenzsegment wird voraussichtlich zwischen 2025 und 2032 das schnellste Wachstum verzeichnen, getrieben durch die rapide alternde Weltbevölkerung und die zunehmende Prävalenz von Alzheimer und verwandten Erkrankungen. Angesichts der steigenden Kosten der Demenzversorgung stehen die Gesundheitssysteme unter Druck, Biosimilars bieten eine kostengünstige Alternative für die Langzeitbehandlung. Pharmaunternehmen entwickeln aktiv monoklonale Antikörper-Biosimilars, die auf die mit Demenz assoziierten Beta-Amyloid- und Tau-Proteine abzielen. Darüber hinaus finden Frühdiagnoseverfahren in Kombination mit Biosimilar-basierten Therapien zunehmend klinische Anwendung. Das Wachstum des Segments wird durch politische Initiativen und Forschungsgelder im Bereich neurodegenerativer Erkrankungen zusätzlich unterstützt.
- Nach Indikation
Der Biosimilar-Markt ist nach Indikation segmentiert in Vorhofseptumdefekt (ASD), Ventrikelseptumdefekt (VSD), persistierendes Foramen ovale (PFO), Aortenklappenstenose und weitere. Das Segment Aortenklappenstenose dominierte den Markt im Jahr 2024 aufgrund der hohen Prävalenz von Herz-Kreislauf-Erkrankungen und des Bedarfs an erschwinglichen, biobasierten Interventionen. Biosimilars tragen dazu bei, die Gesamtkosten für Klappenersatz und damit verbundene Therapien zu senken und die Behandlungen zugänglicher zu machen. Die zunehmende Akzeptanz in Industrie- und Schwellenländern stärkt diese Dominanz. Krankenhäuser bevorzugen biosimilarbasierte Interventionen aufgrund der nachgewiesenen Wirksamkeit und Kosteneinsparungen bei großen Patientenpopulationen.
Das Segment der Ventrikelseptumdefekte (VSD) wird voraussichtlich zwischen 2025 und 2032 aufgrund der steigenden Zahl angeborener Herzfehler bei Säuglingen und Kindern am schnellsten wachsen. Biosimilars werden aufgrund ihrer Erschwinglichkeit und Verfügbarkeit zunehmend in Behandlungsprotokolle integriert. Verbesserungen in der pädiatrischen Herzversorgung und steigende Gesundheitsausgaben in Schwellenländern beschleunigen die Akzeptanz. Darüber hinaus trägt die zunehmende Zusammenarbeit zwischen Biosimilar-Entwicklern und Kinderkliniken zur Erweiterung des Behandlungsangebots bei. Auch zunehmende Aufklärungskampagnen zu angeborenen Herzfehlern tragen zu diesem Wachstum bei.
- Nach Therapietyp
Der Biosimilar-Markt ist nach Therapieformen in die Bereiche Onkologie, Immunologie, Hämatologie, Hormontherapie, Stoffwechselerkrankungen und weitere unterteilt. Das Onkologie-Segment dominierte den Markt im Jahr 2024 mit dem größten Umsatzanteil von 42,2 %, unterstützt durch den weit verbreiteten Einsatz von monoklonalen Antikörper-Biosimilars in der Krebsbehandlung. Die weltweit steigende Krebsbelastung und die hohen Kosten für Marken-Biologika treiben die Akzeptanz von Biosimilars voran. Regulierungsbehörden haben die Zulassungsverfahren für onkologische Biosimilars priorisiert, um Patienten einen schnelleren Zugang zu ermöglichen. Krankenhäuser und Krebszentren integrieren Biosimilars rasch in ihre Behandlungsprotokolle, um die Kostenerschwinglichkeit und den Zugang zu verbessern. Die wachsende Pipeline an onkologischen Biosimilars stärkt die starke Marktposition dieses Segments weiter.
Das Segment Immunologie dürfte zwischen 2025 und 2032 das schnellste Wachstum verzeichnen, angetrieben durch die zunehmende Verbreitung von Autoimmunerkrankungen wie rheumatoider Arthritis, Psoriasis und entzündlichen Darmerkrankungen. Biosimilars bieten kostengünstige Alternativen zu teuren Biologika wie TNF-Inhibitoren und IL-gerichteten Therapien. Fördernde regulatorische Rahmenbedingungen, die die Substitution in der Immunologie fördern, stärken das Segment zusätzlich. Die Nachfrage der Patienten nach langfristigen, kostengünstigen Behandlungsmöglichkeiten unterstützt ebenfalls eine schnelle Akzeptanz. Dank aussagekräftiger klinischer Daten zur Wirksamkeit gewinnen immunologische Biosimilars bei Ärzten zunehmend an Akzeptanz.
- Von Endbenutzern
Der Biosimilar-Markt ist nach Endverbrauchern in Krankenhäuser, Kliniken, Diagnosezentren und andere Bereiche unterteilt. Krankenhäuser dominierten den Markt im Jahr 2024 aufgrund ihrer Rolle als primäre Zentren für die Verabreichung von Biosimilar-Therapien in den Bereichen Onkologie, Kardiologie und Neurologie. Krankenhäuser profitieren von ihrer großen Einkaufsmacht und etablierten Partnerschaften mit Biosimilar-Herstellern, wodurch sie die Behandlungskosten deutlich senken können. Staatlich finanzierte Krankenhausprogramme und Versicherungserstattungsrichtlinien fördern die Einführung von Biosimilars in diesen Bereichen zusätzlich. Darüber hinaus verfügen Krankenhäuser über die notwendige Infrastruktur und das Fachwissen für den sicheren Umgang mit komplexen Biologika.
Das Kliniksegment wird voraussichtlich zwischen 2025 und 2032 das schnellste Wachstum verzeichnen, angetrieben durch den zunehmenden Trend zur ambulanten Versorgung und den Ausbau von Spezialkliniken für das Management chronischer Krankheiten. Kliniken setzen zunehmend Biosimilars ein, da diese kostengünstiger sind und sich leicht in Routinebehandlungsprotokolle integrieren lassen. Patienten bevorzugen Kliniken aufgrund ihrer Erreichbarkeit, kürzerer Wartezeiten und einer personalisierten Betreuung. Darüber hinaus spielen Kliniken eine wichtige Rolle bei der Ausweitung des Zugangs zu Biosimilars in Vorstädten und ländlichen Regionen. Partnerschaften zwischen Biosimilar-Entwicklern und Kliniknetzwerken treiben die Akzeptanz weiter voran.
Regionale Analyse des Biosimilar-Marktes
- Nordamerika dominierte den globalen Biosimilar-Markt mit dem größten Umsatzanteil von 42,8 % im Jahr 2024, was auf die starke regulatorische Unterstützung durch die US-amerikanische FDA, den zunehmenden Eintritt von Biosimilars in hochwertige therapeutische Kategorien und die verstärkte Akzeptanz bei Kostenträgern zurückzuführen ist, die nach erschwinglichen Alternativen zu teuren Biologika suchen.
- Der gut etablierte Regulierungsrahmen der Region, insbesondere das Zulassungsverfahren für Biosimilars der FDA, hat einen starken Markteintritt begünstigt und Innovationen bei den Pharmaherstellern gefördert.
- Hohe Gesundheitsausgaben, eine fortschrittliche Gesundheitsinfrastruktur und ein starker Versicherungsschutz unterstützen die schnelle Akzeptanz von Biosimilars als kostengünstige Alternative zu Marken-Biologika.
Einblicke in den US-amerikanischen Biosimilar-Markt
Der US-Biosimilar-Markt erzielte 2024 mit 83 % den größten Umsatzanteil innerhalb Nordamerikas. Begünstigt wird dies durch die starke Nachfrage nach kostengünstigen biologischen Alternativen und die rasante Ausweitung therapeutischer Anwendungen in der Onkologie und Immunologie. Ärzte und Patienten setzen zunehmend auf Biosimilars, da diese nachweislich sicher und wirksam sind und im Vergleich zu Marken-Biologika erhebliche Kosteneinsparungen bieten. Die positive regulatorische Unterstützung durch die FDA und die wachsende Akzeptanz bei den Versicherern beschleunigen die Marktdurchdringung in Krankenhäusern und Fachkliniken. Darüber hinaus tragen der Markteintritt großer Pharmaunternehmen und die erweiterte Kostenerstattung maßgeblich zum Marktwachstum bei.
Einblicke in den europäischen Biosimilar-Markt
Der europäische Biosimilar-Markt wird im Prognosezeitraum voraussichtlich mit einer deutlichen jährlichen Wachstumsrate wachsen, getrieben durch etablierte regulatorische Rahmenbedingungen und die frühzeitige Einführung wichtiger Therapieklassen. Länder wie Deutschland, Frankreich und Großbritannien sind aufgrund starker staatlicher Initiativen zur Senkung der Gesundheitsausgaben führend bei der Nutzung von Biosimilars. Die steigende Prävalenz chronischer Erkrankungen und der Wunsch nach erschwinglichen Behandlungen fördern die Akzeptanz in der Onkologie, Endokrinologie und Rheumatologie. Das wachsende Vertrauen der Ärzte und wettbewerbsfähige Preisstrategien fördern zudem die Verbreitung von Biosimilars in Krankenhäusern und Apotheken.
Einblicke in den britischen Biosimilar-Markt
Der britische Biosimilar-Markt wird im Prognosezeitraum voraussichtlich mit einer bemerkenswerten jährlichen Wachstumsrate wachsen, unterstützt durch die positive NHS-Politik, die die Einführung von Biosimilars aktiv fördert. Gesundheitsdienstleister setzen aufgrund des Kostendrucks und der Notwendigkeit eines breiteren Patientenzugangs zunehmend auf Biosimilars in den Bereichen Onkologie, Immunologie und Stoffwechselerkrankungen. Die wachsende Akzeptanz bei Verschreibern und Patienten, kombiniert mit wettbewerbsfähigen Ausschreibungen und Preissenkungen, dürfte die Marktdurchdringung beschleunigen. Darüber hinaus stärken Kooperationen zwischen dem NHS und Biosimilar-Herstellern das Vertrauen und fördern die weitere Einführung.
Einblicke in den deutschen Biosimilar-Markt
Der deutsche Biosimilar-Markt wird im Prognosezeitraum voraussichtlich mit einer beträchtlichen jährlichen Wachstumsrate wachsen, was auf die Position Deutschlands als einer der ersten Anwender von Biosimilars in Europa zurückzuführen ist. Eine starke Gesundheitspolitik, die Substitution fördert, gepaart mit wettbewerbsfähigen Preisen, hat Deutschland zu einem führenden Anbieter von Biosimilars in allen Therapiebereichen gemacht. Der Fokus auf die Senkung der Gesundheitskosten und die Verbesserung des Zugangs zu Biologika unterstützt die schnelle Akzeptanz in der Onkologie und bei Autoimmunerkrankungen. Darüber hinaus verstärken lokale Innovationen, Aufklärungsprogramme für Ärzte und strukturierte Erstattungsrahmen das Marktwachstum.
Einblicke in den Biosimilar-Markt im asiatisch-pazifischen Raum
Der Biosimilar-Markt im asiatisch-pazifischen Raum wird im Prognosezeitraum 2025 bis 2032 voraussichtlich mit einer durchschnittlichen jährlichen Wachstumsrate von 25 % wachsen. Dies ist auf die zunehmende Belastung durch chronische Krankheiten, den Ausbau der Gesundheitsinfrastruktur und die zunehmende Erschwinglichkeit von Biosimilars in Ländern wie China, Japan und Indien zurückzuführen. Fördernde staatliche Initiativen und lokale Produktionskapazitäten machen Biosimilars einem breiteren Patientenkreis zugänglicher. Strategische Kooperationen zwischen nationalen und internationalen Akteuren fördern zudem Innovationen und sichern behördliche Zulassungen. Die wachsende Mittelschicht in der Region und steigende Investitionen im Gesundheitswesen beschleunigen die Einführung von Biosimilars zusätzlich.
Einblicke in den japanischen Biosimilar-Markt
Der japanische Biosimilar-Markt gewinnt dank starker staatlicher Unterstützung, des Bedarfs der alternden Bevölkerung und der steigenden Prävalenz von Krebs und Autoimmunerkrankungen an Dynamik. Die japanischen Regulierungsbehörden haben die Zulassungsverfahren vereinfacht und so die Verfügbarkeit von Biosimilars in allen Therapiebereichen erhöht. Die zunehmende Akzeptanz in Krankenhäusern und Fachkliniken sowie das wachsende Vertrauen der Ärzte kurbeln die Nachfrage an. Darüber hinaus fördern die fortschrittliche Gesundheitsinfrastruktur des Landes und der Fokus auf Kostensenkung eine breitere Marktakzeptanz. Partnerschaften zwischen japanischen und globalen Biopharmaunternehmen unterstützen die Marktexpansion zusätzlich.
Einblicke in den indischen Biosimilar-Markt
Der indische Biosimilar-Markt hatte 2024 den größten Marktanteil im asiatisch-pazifischen Raum, unterstützt durch ein robustes inländisches Produktionsökosystem und eine wachsende Nachfrage nach erschwinglichen Biologika. Indien hat sich als globales Zentrum für die Entwicklung und Produktion von Biosimilars positioniert und beliefert sowohl den nationalen als auch den internationalen Markt. Steigende Fälle von Diabetes, Krebs und Autoimmunerkrankungen fördern die Akzeptanz in allen Therapiebereichen. Darüber hinaus treiben staatliche Initiativen zur Verbesserung der Gesundheitsversorgung sowie die Präsenz führender lokaler Akteure die starke Marktdurchdringung von Biosimilars voran. Wettbewerbsfähige Preisstrategien und das wachsende Vertrauen der Ärzte treiben das Marktwachstum weiter voran.
Marktanteil von Biosimilars
Die Biosimilar-Branche wird hauptsächlich von etablierten Unternehmen angeführt, darunter:
- Novartis AG (Schweiz)
- Orion Pharma AB (Schweden)
- Pfizer Inc. (USA)
- Samsung Bioepis. (Südkorea)
- Coherus BioSciences, Inc. (USA)
- Amgen Inc. (USA)
- Lilly USA, LLC (USA)
- Takeda Pharmaceutical Company Limited. (Japan)
- Bristol-Myers Squibb Company (USA)
- Merck KGaA (Deutschland)
- Teva Pharmaceutical Industries Ltd. (USA)
- Biocon (Indien)
- Bayer AG (Deutschland)
- AbbVie Inc. (USA)
- Dr. Reddy's Laboratories Ltd. (Indien)
- Boehringer Ingelheim International GmbH (Deutschland)
- Biogen (USA)
Was sind die jüngsten Entwicklungen auf dem globalen Biosimilar-Markt?
- Im Mai 2025 genehmigte die FDA Starjemza (Ustekinumab-hmny) als achtes Biosimilar zu Stelara (Ustekinumab) und bietet Patienten verbesserte Behandlungsmöglichkeiten für rheumatische und gastrointestinale Erkrankungen. Diese Zulassung unterstreicht die kontinuierliche Expansion der Ustekinumab-Biosimilar-Klasse und unterstützt einen besseren Zugang zu diesen Therapien.
- Im Februar 2025 stufte die FDA Selarsdi, ein Biosimilar zu Stelara (Ustekinumab), als austauschbar ein. Das bedeutet, dass Apotheker es nach Ablauf der Exklusivitätsfristen ohne Eingriff des verschreibenden Arztes durch Stelara ersetzen können, was den Zugang und die Akzeptanz für Patienten erheblich vereinfacht.
- Im Februar 2025 genehmigte die FDA Merilog (Insulin-Aspart-szjj), das erste schnell wirkende Insulin-Biosimilar zu Novolog (Insulin-Aspart), das sowohl als Fertigpen als auch als Ampulle erhältlich ist. Dies ist ein Meilenstein bei der Erweiterung erschwinglicher Insulinoptionen für Diabetespatienten.
- Im Dezember 2024 erteilte die FDA Steqeyma (Ustekinumab-stba) die Zulassung als siebtes Biosimilar zu Stelara (Ustekinumab) und ermöglichte damit mehr Wettbewerb und erweiterte Behandlungsmöglichkeiten bei Autoimmun- und Entzündungskrankheiten.
- Im Oktober 2024 gab Accord BioPharma, Inc. bekannt, dass die FDA Imuldosa (Ustekinumab-srlf), ein Biosimilar zu Stelara (Ustekinumab), für alle gleichen chronischen Entzündungsindikationen zugelassen hat – darunter Psoriasis, Psoriasis-Arthritis, Morbus Crohn und Colitis ulcerosa.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.