Europe Prescription Digital Therapeutics Dtx Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
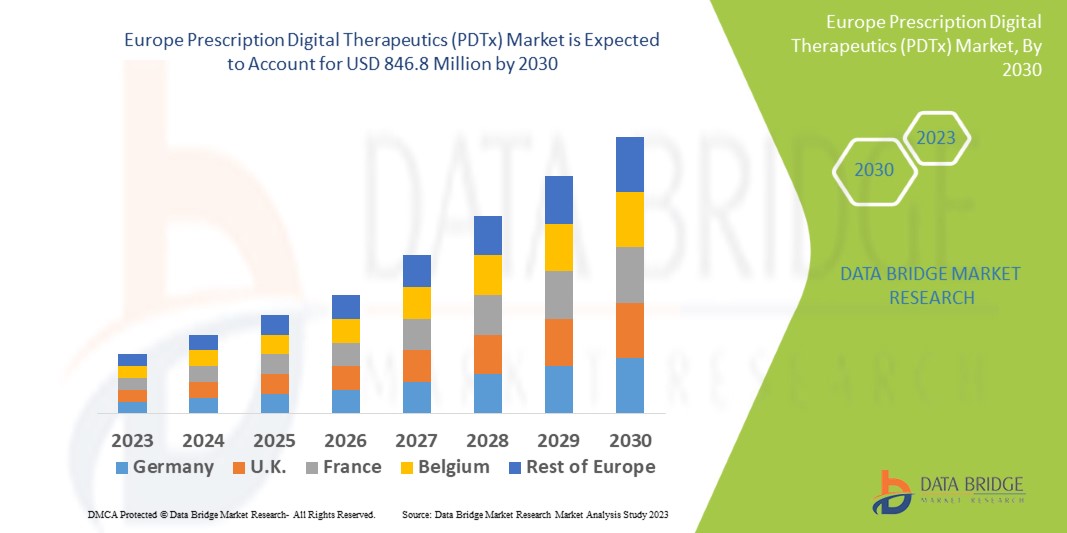
179.50 Million
USD
846.80 Million
2022
2030
USD
179.50 Million
USD
846.80 Million
2022
2030
| 2023 –2030 | |
| USD 179.50 Million | |
| USD 846.80 Million | |
|
|
|
|
Europe Prescription Digital Therapeutics (PDTx) Market, By Mechanism (Input Mechanisms, Output Mechanisms), Category (Medication Augmentation, Medication Replacement), Treatment (Outpatient Treatment, Monotherapy), Software (Software For Respiratory Conditions, Software For Mental Health, Software For Opioid Use Disorder, Software For Diabetes, Others), Services (Behavioral Microservices, Medical Microservices), App Accessibility (Android, iOS, Windows), App Type (Native Apps, Web Apps), Application (Substance Use Disorder (SUD), Opioid Use Disorder (OUD), Attention Deficit/Hyperactivity Disorder (ADHD), Alzheimer’s Disease, Major Depressive Disorder (MDD), Insomnia, Epilepsy, Movement Disorder, Multiple Sclerosis, Migraine, Autism Spectrum Disorder, Oncology, Inflammation, Respiratory, Cardiovascular, Pain Management, Metabolic Conditions, Others), Patients (Children, Adults) – Industry Trends and Forecast to 2030.
Europe Prescription Digital Therapeutics (PDTx) Market Analysis and Size
Prescription digital therapeutics have been developed as a boon for a number of diseases such as substance use disorder (SUD), ADHD, major depressive disorder (MDD), opioid use disorder (OUD), insomnia, epilepsy, multiple sclerosis, autism spectrum disorder, and cardiovascular disease. Patients with several unmet requirements may find prescription digital therapies to be the most vital aspect of their lives. Presently, numerous research & clinical studies are going on which is anticipated to create a competitive advantage for several manufacturers to develop new and innovative technology.
Data Bridge Market Research analyses a growth rate in the prescription digital therapeutics (PDTx) market in the forecast period 2023-2030. The expected CAGR of prescription digital therapeutics (PDTx) market is tend to be around 21.4% in the mentioned forecast period. The market value is USD 179.5 million in 2022, and it would grow upto USD 846.8 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Prescription Digital Therapeutics (PDTx) Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Mechanismus (Eingabemechanismen, Ausgabemechanismen), Kategorie (Medikamentenergänzung, Medikamentenersatz), Behandlung (ambulante Behandlung, Monotherapie), Software (Software für Atemwegserkrankungen, Software für psychische Gesundheit, Software für Opioidkonsumstörungen, Software für Diabetes, Sonstige), Dienste (Verhaltens-Mikrodienste, Medizinische Mikrodienste), App-Zugänglichkeit (Android, iOS, Windows), App-Typ (Native Apps, Web-Apps), Anwendung (Substanzkonsumstörung (SUD), Opioidkonsumstörung (OUD), Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung (ADHS), Alzheimer-Krankheit, Major Depression (MDD), Schlaflosigkeit, Epilepsie, Bewegungsstörung, Multiple Sklerose, Migräne, Autismus-Spektrum-Störung, Onkologie, Entzündung, Atemwege, Herz-Kreislauf, Schmerzbehandlung, Stoffwechselerkrankungen, Sonstige), Patienten (Kinder, Erwachsene) |
|
Abgedeckte Länder |
Deutschland, Frankreich, Großbritannien, Niederlande, Schweiz, Belgien, Russland, Italien, Spanien, Türkei, Restliches Europa in Europa |
|
Abgedeckte Marktteilnehmer |
ResMed (USA), SAMSUNGHEALTHCARE (Südkorea), Biofourmis (USA), Novartis AG (Schweiz), Medtronic (Irland), Pear Therapeutics, Inc. (USA), Voluntis (Frankreich), Omada Health, Inc. (USA), GAIA AG (Deutschland), Welldoc's Bluestar (USA), Solera Network (USA), Akili Interactive Labs, Inc. (USA), Better Therapeutics, LLC (USA), BigHealth (USA), Biofourmis (USA), Click Therapeutics, Inc. (USA), Happify, Inc. (USA), Limbix Health, Inc. (USA), Naturalcycles Nordic AB (Schweden), NuvoAir AB (Schweden), Sensyne Health plc. (Großbritannien), Xealth (USA) |
|
Marktchancen |
|
Marktdefinition
Verschreibungspflichtige digitale Therapien sind eine Art Software, die zur Behandlung zahlreicher Beschwerden eingesetzt wird. Künstliche Intelligenz, verschiedene Algorithmen und virtuelle Realität werden häufig zur Entwicklung dieser therapeutischen Instrumente eingesetzt. Patienten nutzen manchmal verschreibungspflichtige digitale Therapiesoftware und -dienste, um jede Form der Behandlung zu erhalten, die sie benötigen. Diese Medikamente werden von der FDA und anderen Aufsichtsbehörden streng reguliert und können nur mit einem entsprechenden Rezept eines Arztes verwendet werden.
Marktdynamik für verschreibungspflichtige digitale Therapeutika (PDTx) in Europa
Treiber
- Zunehmende Zahl chronischer Erkrankungen
Die zunehmende Verbreitung chronischer Krankheiten ist ein Hauptfaktor, der die Wachstumsrate des Marktes für verschreibungspflichtige digitale Therapeutika (PDTx) im Prognosezeitraum ankurbelt. Laut der Weltgesundheitsorganisation (WHO) werden im Jahr 2020 rund 463 Millionen Erwachsene an Diabetes erkrankt sein. Im Jahr 2021 berichteten 35,2 % der europäischen Bevölkerung ab 16 Jahren von einer Langzeiterkrankung. Auch die Zahl der Menschen, die Krebs überleben, ist gestiegen. Derzeit befinden sich voraussichtlich 16–17 Millionen europäische Bürger entweder in der Behandlungsphase gegen Krebs oder in der Nachbehandlungsphase, und diese Zahl wird in den nächsten 10–20 Jahren ebenfalls deutlich steigen. Daher steigert dieser Faktor das Wachstum des Marktes.
- Entwicklung einer Pipeline für potenzielle Produkte
Eine große Pipeline potenzieller Produkte, die voraussichtlich im Prognosezeitraum auf den Markt kommen, dürfte das Wachstum verschreibungspflichtiger digitaler Therapeutika (PDTx) fördern. Beispielsweise sind Pear-011 (Angstzustände, generalisierte Angststörung), Pear-015 (Depression, MDD), die zur Behandlung von Erwachsenen mit chronischer Schlaflosigkeit und Depression eingesetzt werden, und CT-155 (Schizophrenie), CT-152 (schwere depressive Störung), die zur Behandlung von Erwachsenen mit Depressionen und Schizophrenie eingesetzt werden, Produkte potenzieller klinischer Therapeutika von Pear Therapeutics in Phase III. Dieser Faktor fördert das Marktwachstum.
Gelegenheiten
- Verstärkte Forschungsaktivitäten
Die Forschungsaktivitäten im Zusammenhang mit verschreibungspflichtigen digitalen Therapeutika nehmen zu und fördern so das Marktwachstum. So wurde beispielsweise NuvoAir AB im August 2020 als Medizinprodukt der Klasse I zertifiziert. Das Unternehmen hat diese Zertifizierung aufgrund seiner fortschrittlichen Funktionen erhalten, darunter ein zertifiziertes Bluetooth-Spirometer in Kombination mit dem Gesundheitsportal und der angeschlossenen Patienten-App. Diese Zertifizierung hat den Markenwert bei Ärzten und Patienten gesteigert. Somit hat dieser Faktor viele Möglichkeiten für das Marktwachstum geschaffen.
- Zunehmende Nutzung von Software für Diabetes
Man geht davon aus, dass der Markt für Diabetessoftware wachsen wird, da weltweit ein großer Teil der Bevölkerung an Diabetes leidet und Diabetes die häufigste Todesursache ist. Einer Umfrage zufolge leiden 463 Millionen Menschen an Diabetes und diese Zahl wächst kontinuierlich und rasant. Aus diesem Grund steigen auch die Gesundheitsausgaben für Diabetes in Europa und es wird viel geforscht, um neue und einfache Behandlungsmöglichkeiten für die Patienten zu finden. Daher trägt dieser Faktor dazu bei, viele Möglichkeiten für das Wachstum des Marktes zu schaffen.
Einschränkungen/Herausforderungen
- Sicherheit und Datenmanagement
PDTx überträgt Informationen über das Internet, daher könnte das Risiko eines unbefugten Zugriffs und einer Manipulation dieser Lösungen das Produktvertrauen und die Patientenversorgung gefährden. Die FDA hat Richtlinien zu Cybersicherheit, Kennzeichnung und Dokumentation für Zulassungsanträge vor der Markteinführung herausgegeben, aber mehrere Behörden verlangen derzeit keine Sicherheitsprüfungen für Medizinprodukte vor der Markteinführung. Es liegt in der alleinigen Verantwortung der Gerätehersteller, das mit ihren Produkten verbundene Cybersicherheitsrisiko zu definieren und Maßnahmen zur Risikominderung zu ergreifen. Dies behindert das Marktwachstum.
- Schwierigkeiten bei der Anpassung digitaler Therapeutika durch Ärzte
Nicht alle Ärzte und Anbieter sind mit der zunehmenden Digitalisierung vertraut. Ärzte und medizinisches Fachpersonal sollten sich mit digitalen Therapielösungen vertraut machen, bevor sie diese massenhaft einsetzen. Sie sollten die Ergebnisse klinischer Studien analysieren, bevor sie ihren Patienten die Lösungen empfehlen. Um dies zu erreichen, entscheiden sich einige DTx-Unternehmen für ein pharmazeutisches Modell, bei dem Partnerschaften mit Vertriebshändlern genutzt werden, um Ärzte über Ergebnisse klinischer Studien, neue Lösungen und Entscheidungen zu informieren. Dies behindert also das Marktwachstum.
Dieser Marktbericht für verschreibungspflichtige digitale Therapeutika (PDTx) enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-Export-Analysen, Produktionsanalysen, Wertschöpfungskettenoptimierungen, Marktanteilen, Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neue Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um weitere Informationen zum Markt für verschreibungspflichtige digitale Therapeutika (PDTx) zu erhalten, wenden Sie sich an Data Bridge Market Research, um einen Analystenbericht zu erhalten. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Jüngste Entwicklung
- Im Jahr 2020 gab Biofourmis bekannt, dass seine Biovitals-Plattform vom Gesundheitsministerium (MOH) in Singapur in großem Umfang genutzt wurde, um den Gesundheitszustand von COVID-19-Patienten zu überwachen. Diese Initiative ermöglichte es den Krankenschwestern und Ärzten, die Krankheitssymptome frühzeitig zu diagnostizieren, sodass die Patienten eine wirksame Behandlung erhalten konnten. Somit verbesserte diese Initiative den Umsatz des Unternehmens, indem sie die Akzeptanzrate während der COVID-19-Pandemie erhöhte.
Marktumfang für verschreibungspflichtige digitale Therapeutika (PDTx) in Europa
Der Markt für verschreibungspflichtige digitale Therapeutika (PDTx) ist segmentiert nach Mechanismus, Kategorie, Behandlung, Software, Diensten, App-Zugänglichkeit, App-Typ, Anwendung und Patienten. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, die ihnen bei der strategischen Entscheidungsfindung zur Identifizierung der wichtigsten Marktanwendungen helfen.
Mechanismus
- Eingabemechanismen
- Ausgabemechanismen
Kategorie
- Medikamentenergänzung
- Medikamentenersatz
Behandlung
- Ambulante Behandlung
- Monotherapie
Software
- Software für Atemwegserkrankungen
- Software für die psychische Gesundheit
- Software für Opioidkonsumstörungen
- Software für Diabetes
- Sonstiges
Dienstleistungen
- Verhaltensbasierte Microservices
- Medizinische Microservices
App-Zugänglichkeit
- Android
- iOS
- Windows
App-Typ
- Native Apps
- Web-Apps
Anwendung
- Substanzgebrauchsstörung (Substance Use Disorder, SUD)
- Opioidkonsumstörung (OUD)
- Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung (ADHS)
- Alzheimer-Krankheit
- Schwere depressive Störung (MDD)
- Schlaflosigkeit
- Epilepsie
- Bewegungsstörung
- Multiple Sklerose
- Migräne
- Autismus-Spektrum-Störung
- Onkologie
- Entzündung
- Atemwege
- Herz-Kreislauf
- Schmerztherapie
- Stoffwechselerkrankungen
- Sonstiges
Patienten
- Kinder
- Erwachsene
Regionale Analyse/Einblicke zum Markt für verschreibungspflichtige digitale Therapeutika (PDTx)
Der Markt für verschreibungspflichtige digitale Therapeutika (PDTx) wird analysiert und Einblicke in die Marktgröße und Trends werden nach Mechanismus, Kategorie, Behandlung, Software, Diensten, App-Zugänglichkeit, App-Typ, Anwendung und Patienten wie oben angegeben bereitgestellt.
Die wichtigsten im Marktbericht für verschreibungspflichtige digitale Therapeutika (PDTx) abgedeckten Länder sind Deutschland, Frankreich, Großbritannien, Niederlande, Schweiz, Belgien, Russland, Italien, Spanien, Türkei und das übrige Europa.
Deutschland wird im Prognosezeitraum voraussichtlich die höchste durchschnittliche jährliche Wachstumsrate aufweisen, da die Anwendung verschreibungspflichtiger digitaler Therapeutika in mehreren europäischen Ländern mit dem technologischen Fortschritt in der Software zunimmt. Darüber hinaus fördert auch die zunehmende Zahl inländischer Unternehmen, die auf dem Markt tätig sind, das Marktwachstum.
Der Länderabschnitt des Berichts enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Regulierung auf dem Inlandsmarkt, die sich auf die aktuellen und zukünftigen Markttrends auswirken. Außerdem werden die Präsenz und Verfügbarkeit europäischer Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen inländischer Zölle und Handelsrouten berücksichtigt, während eine Prognoseanalyse der Länderdaten bereitgestellt wird.
Wettbewerbsumfeld und Marktanteilsanalyse für verschreibungspflichtige digitale Therapeutika (PDTx) in Europa
Die Wettbewerbslandschaft auf dem Markt für verschreibungspflichtige digitale Therapeutika (PDTx) bietet Details nach Wettbewerbern. Zu den Details gehören Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, Präsenz in Europa, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang, Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen in Bezug auf den Markt für verschreibungspflichtige digitale Therapeutika (PDTx).
Zu den wichtigsten Akteuren auf dem Markt für verschreibungspflichtige digitale Therapeutika (PDTx) gehören:
- ResMed (USA)
- SAMSUNGHEALTHCARE (Südkorea)
- Biofourmis (USA)
- Novartis AG (Schweiz)
- Medtronic (Irland)
- Pear Therapeutics, Inc. (USA)
- Voluntis (Frankreich)
- Omada Health, Inc. (USA)
- GAIA AG (Deutschland)
- Welldocs Bluestar (USA)
- Solera-Netzwerk (USA)
- Akili Interactive Labs, Inc. (USA)
- Better Therapeutics, LLC (USA)
- BigHealth (USA)
- Biofourmis (USA)
- Click Therapeutics, Inc. (USA)
- Happify, Inc. (USA)
- Limbix Health, Inc. (USA)
- Naturalcycles Nordic AB (Schweden)
- NuvoAir AB (Schweden)
- Sensyne Health plc. (Großbritannien)
- Xealth (USA)
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.