Europe Anesthesia And Respiratory Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
0.99 Million
USD
2.00 Million
2024
2032
USD
0.99 Million
USD
2.00 Million
2024
2032
| 2025 –2032 | |
| USD 0.99 Million | |
| USD 2.00 Million | |
|
|
|
|
Europäischer Markt für Anästhesie- und Beatmungsgeräte, nach Produkttyp (Anästhesiegeräte, Beatmungsgeräte, Überwachungsgeräte, Diagnosegeräte, Verbrauchsmaterialien und Zubehör) nach Endbenutzer (Krankenhäuser, Kliniken, häusliche Pflegeeinrichtungen, ambulante Servicezentren), Land (Deutschland, Italien, Großbritannien, Frankreich, Spanien, Niederlande, Belgien, Schweiz, Türkei, Russland, übriges Europa) – Branchentrends und Prognose bis 2032
Marktgröße für Anästhesie- und Beatmungsgeräte
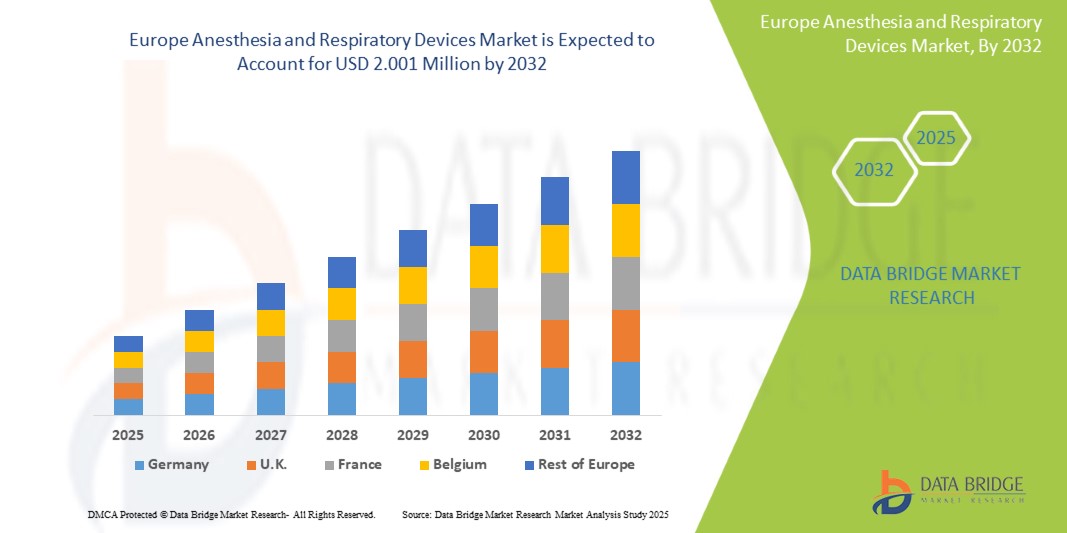
- Der europäische Markt für Anästhesie- und Beatmungsgeräte wurde im Jahr 2024 auf 0,986 Millionen US-Dollar geschätzt und soll bis 2032 2,001 Millionen US-Dollar erreichen.
- Im Prognosezeitraum von 2025 bis 2032 wird der Markt voraussichtlich mit einer jährlichen Wachstumsrate von 6,35 % wachsen, was vor allem auf die zunehmende Verbreitung von Atemwegserkrankungen, die alternde Bevölkerung und die steigende Zahl chirurgischer Eingriffe in der gesamten Region zurückzuführen ist.
- Das Wachstum wird zusätzlich durch technologische Fortschritte bei Anästhesie- und Beatmungssystemen, steigende Gesundheitsausgaben und die zunehmende Nutzung tragbarer und für die häusliche Pflege geeigneter Beatmungsgeräte im Zuge eines wachsenden Trends zur personalisierten Pflege unterstützt.
Marktanalyse für Anästhesie- und Beatmungsgeräte
- Anästhesie- und Beatmungsgeräte sind wichtige Komponenten der perioperativen Versorgung und des Managements chronischer Krankheiten und werden in Krankenhäusern, ambulanten Operationszentren und in der häuslichen Pflege häufig eingesetzt.
- Das Produktportfolio umfasst Anästhesiegeräte, Beatmungsgeräte, Vernebler, CPAP/BiPAP-Geräte, Sauerstoffkonzentratoren und Einweg-Beatmungsgeräte, die alle für eine präzise und kontrollierte Atemunterstützung bei Operationen oder chronischer Atemtherapie unerlässlich sind.
- Die Nachfrage nach diesen Geräten wird durch die steigende Zahl chronischer Atemwegserkrankungen wie COPD und Schlafapnoe, die nach der Pandemie verstärkte Betonung der Atemwegsgesundheit und eine Verlagerung hin zu nicht-invasiver Beatmung und Atemtherapielösungen für zu Hause angeheizt.
- Darüber hinaus tragen Europas starker Fokus auf Innovationen im Bereich medizinischer Geräte, unterstützende regulatorische Rahmenbedingungen und die wachsende Zahl älterer Menschen zur Marktexpansion bei.
Berichtsumfang und Marktsegmentierung für Anästhesie- und Beatmungsgeräte
|
Eigenschaften |
Wichtige Markteinblicke zu Anästhesie- und Beatmungsgeräten |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Europa
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für Anästhesie- und Beatmungsgeräte
„Steigende Nachfrage nach tragbaren und häuslichen Lösungen zur Beatmungstherapie“
- Es gibt einen wachsenden Trend hin zu tragbaren, kompakten und benutzerfreundlichen Beatmungs- und Anästhesiegeräten, der auf die alternde Bevölkerung, die steigende Zahl chronischer Atemwegserkrankungen und die steigende Nachfrage nach häuslicher Gesundheitsversorgung zurückzuführen ist.
- Technologische Fortschritte ermöglichen die Entwicklung leichter CPAP/BiPAP-Geräte, tragbarer Sauerstoffkonzentratoren und batteriebetriebener Vernebler, die die Mobilität und den Komfort der Patienten verbessern.
- So brachte Philips beispielsweise im Februar 2024 eine verbesserte Version seines tragbaren BiPAP-Geräts mit erweiterten Konnektivitätsfunktionen für Echtzeitüberwachung und Telemedizin-Unterstützung auf den Markt.
- Dieser Trend steht im Einklang mit der allgemeinen Verlagerung hin zu personalisierter Pflege, Fernüberwachung von Patienten und weniger Wiedereinweisungen ins Krankenhaus, insbesondere in Ländern mit fortschrittlichen Gesundheitssystemen wie Deutschland, Frankreich und Großbritannien.
Marktdynamik für Anästhesie- und Beatmungsgeräte
Treiber
„Zunehmende Belastung durch chronische Atemwegserkrankungen und chirurgische Eingriffe“
- Die zunehmende Verbreitung chronischer Atemwegserkrankungen wie COPD, Asthma und Schlafapnoe treibt die Nachfrage nach Geräten zur Atemunterstützung in ganz Europa deutlich an
- Darüber hinaus erhöht die steigende Zahl von Operationen, insbesondere bei alternden Bevölkerungen, den Bedarf an zuverlässigen und effizienten Anästhesiesystemen.
- Laut Eurostat-Daten aus dem Jahr 2023 stieg beispielsweise die Zahl der in europäischen Krankenhäusern durchgeführten chirurgischen Eingriffe im Vergleich zum Vorjahr um über 8 %, wobei die größten Zuwächse bei orthopädischen und kardiovaskulären Operationen zu verzeichnen waren.
- Die kontinuierlichen Fortschritte in der Beatmungstechnologie, der Anästhesieüberwachung und der Integration in elektronische Gesundheitsakten (EHRs) stärken die Marktakzeptanz weiter
Gelegenheit
„Einführung intelligenter, KI-gestützter Anästhesie- und Beatmungsgeräte“
- Der europäische Gesundheitssektor setzt zunehmend auf KI-integrierte Geräte, die durch Echtzeit-Datenanalyse und Automatisierung die Präzision der Atemtherapie und Anästhesie verbessern.
- Intelligente Beatmungs- und Anästhesiegeräte verfügen jetzt über adaptive Beatmungsmodi, automatische Titration und Ferndiagnose und sind daher ideal für die Intensivstation und die häusliche Pflege.
- Im April 2024 führte GE HealthCare in ausgewählten europäischen Krankenhäusern ein KI-gestütztes Anästhesiesystem ein, das prädiktive Erkenntnisse für Dosisanpassungen bietet und die Patientensicherheit verbessert.
- Diese digitale Transformation eröffnet Herstellern neue Möglichkeiten, Mehrwertlösungen anzubieten, die auf die wachsende Bedeutung intelligenter Krankenhäuser und vernetzter Pflege-Ökosysteme in der Region abgestimmt sind.
Einschränkung/Herausforderung
„Strenge regulatorische Anforderungen und Kostenbeschränkungen“
- Der europäische Markt für Medizinprodukte unterliegt strengen regulatorischen Rahmenbedingungen wie der Medizinprodukteverordnung (MDR), die die Markteinführungszeit und die Compliance-Kosten für Hersteller erhöht.
- Hohe Kapitalinvestitionen in moderne Anästhesie- und Beatmungsgeräte schränken die Akzeptanz in kleinen und mittelgroßen Gesundheitseinrichtungen, insbesondere in Mittel- und Osteuropa, oft ein.
- Laut einem Bericht von MedTech Europe aus dem Jahr 2023 stiegen die durchschnittlichen Kosten für die Einhaltung gesetzlicher Vorschriften für ein neues Atemschutzgerät der Klasse II unter der MDR im Vergleich zu früheren Richtlinien um 35 %.
- Darüber hinaus schränkt die begrenzte Kostenerstattung für Beatmungsgeräte für die häusliche Pflege in bestimmten Ländern den breiteren Zugang zu innovativen Technologien ein und führt zu Unterschieden bei der Marktdurchdringung in der Region.
Marktumfang für Anästhesie- und Beatmungsgeräte
Der Markt ist nach Produkt, Rohstoff und Anwendung segmentiert.
|
Segmentierung |
Untersegmentierung |
|
Nach Produkttyp |
|
|
Nach Endbenutzer |
|
Regionale Analyse des Marktes für Anästhesie- und Beatmungsgeräte
„Deutschland ist die dominierende Region im Markt für Anästhesie- und Beatmungsgeräte“
- Deutschland ist führend auf dem Markt für Anästhesie- und Beatmungsgeräte, unterstützt durch seine hochentwickelte Gesundheitsinfrastruktur, einen starken regulatorischen Rahmen und die steigende Prävalenz von Atemwegs- und chronischen Erkrankungen.
- Die Region zeichnet sich durch eine stark alternde Bevölkerung aus, insbesondere in Ländern wie Deutschland, Frankreich, Italien und Großbritannien. Dies führt zu einer steigenden Nachfrage nach modernen Atemunterstützungs- und Anästhesiesystemen sowohl in Krankenhäusern als auch in der häuslichen Pflege.
- Europäische Regierungen und private Gesundheitsdienstleister investieren zunehmend in hochmoderne medizinische Geräte, um die Behandlungsergebnisse der Patienten zu verbessern und Krankenhausaufenthalte zu verkürzen.
- Darüber hinaus stärkt die Präsenz führender globaler Medizintechnikunternehmen und ein starker Fokus auf medizinische Innovationen und digitale Gesundheitslösungen die Position Europas als dominierender regionaler Markt.
„Frankreich wird voraussichtlich die höchste Wachstumsrate verzeichnen“
- In Frankreich wird das schnellste Wachstum des Marktes für Anästhesie- und Beatmungsgeräte erwartet, angetrieben durch die schnelle Entwicklung der Gesundheitsinfrastruktur, steigende Gesundheitsausgaben und ein zunehmendes Bewusstsein für die Gesundheit der Atemwege.
- Der Anstieg chronischer Atemwegserkrankungen, die Umweltverschmutzung in den Städten und eine wachsende Mittelschicht führen zu einer steigenden Nachfrage nach tragbaren und hochwertigen Beatmungs- und Anästhesiegeräten.
- Darüber hinaus wird erwartet, dass die lokale Fertigung, günstige Regierungsinitiativen und der Eintritt globaler Akteure in regionale Märkte das Wachstum im ganzen Land deutlich vorantreiben werden.
Marktanteil von Anästhesie- und Beatmungsgeräten
Die Wettbewerbslandschaft des Marktes liefert detaillierte Informationen zu den einzelnen Wettbewerbern. Zu den Details gehören Unternehmensübersicht, Unternehmensfinanzen, Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben genannten Datenpunkte beziehen sich ausschließlich auf die Marktausrichtung der Unternehmen.
Regionale Analyse des Marktes für Anästhesie- und Beatmungsgeräte
Markteinblick für Anästhesie- und Beatmungsgeräte in Deutschland
Deutschland war mit einem Umsatzanteil von 24,67 % im Jahr 2024 Marktführer im europäischen Markt für Anästhesie- und Beatmungsgeräte. Dies wurde durch eine ausgereifte Gesundheitsinfrastruktur, ein hohes Operationsvolumen und Innovationen bei Beatmungs- und Anästhesieüberwachungssystemen unterstützt. Die Präsenz weltweit führender Unternehmen wie Drägerwerk und B. Braun fördert die technologische Akzeptanz.
Deutschland dürfte seine Führungsposition behaupten, angetrieben durch staatliche Investitionen in Intensivstationen und die digitale Transformation des Gesundheitswesens. Die steigende Nachfrage nach minimalinvasiver Anästhesie und integrierten Beatmungslösungen unterstützt das weitere Marktwachstum.
Markteinblick in Großbritannien für Anästhesie- und Beatmungsgeräte
Großbritannien dürfte von 2025 bis 2032 mit einer durchschnittlichen jährlichen Wachstumsrate von 9,11 % die höchste Wachstumsrate aufweisen. Dies ist auf den starken Fokus auf digitale Gesundheit, die Erhöhung der Intensivkapazitäten und den Bedarf an Beatmungstherapie nach der Pandemie zurückzuführen. Im Jahr 2024 machte Großbritannien 21,03 % des Marktes aus.
Die schnelle Einführung von KI-gestützter Atemüberwachung und intelligenten Anästhesiearbeitsplätzen, unterstützt durch die Modernisierung des NHS und F&E-Initiativen, positioniert Großbritannien als wichtigen Wachstumsmotor auf dem europäischen Markt.
Markteinblick in Anästhesie- und Beatmungsgeräte in Frankreich
Frankreich hielt im Jahr 2024 einen beachtlichen Marktanteil von 19,58 %, bedingt durch die Zunahme chirurgischer Eingriffe, die alternde Bevölkerung und den Fokus auf die Optimierung der perioperativen Versorgung. Die starke Krankenhausinfrastruktur in Städten wie Lyon und Toulouse unterstützt eine stabile Marktnachfrage.
Der französische Markt wird voraussichtlich mit einer signifikanten jährlichen Wachstumsrate wachsen, angetrieben durch die strategische Beschaffung von Beatmungsgeräten der nächsten Generation und umweltfreundlichen Anästhesiegeräten. Partnerschaften zwischen öffentlichen Krankenhäusern und Geräteherstellern fördern die Technologieintegration.
Die wichtigsten Marktführer auf dem Markt sind:
- Philips Healthcare (Niederlande)
- GE HealthCare (USA)
- Medtronic plc (Irland)
- Drägerwerk AG & Co. KGaA (Deutschland)
- Fisher & Paykel Healthcare Corporation Limited (Neuseeland)
- Smiths Medical (ICU Medical, Inc.) (USA)
- Getinge AB (Schweden)
- Hamilton Medical AG (Schweiz)
- Masimo Corporation (USA)
- Nihon Kohden Corporation (Japan)
- ResMed Inc. (USA)
- Mindray Medical International Limited (China)
- Koninklijke Philips NV (Niederlande)
- Teleflex Incorporated (USA)
- Vyaire Medical, Inc. (USA)
- Schiller AG (Schweiz)
- Siare Engineering International Group (Italien)
- Beurer GmbH (Deutschland)
- Weinmann Emergency Medical Technology GmbH (Deutschland)
Neueste Entwicklungen auf dem europäischen Markt für Anästhesie- und Beatmungsgeräte
- Im Februar 2024 stellte Philips Healthcare ein tragbares BiPAP-Gerät der nächsten Generation mit integrierten KI-Algorithmen zur automatischen Druckanpassung vor, das auf die häusliche Pflege und das Schlafapnoe-Management in ganz Westeuropa ausgerichtet ist.
- Im Januar 2024 erweiterte GE HealthCare sein Portfolio um ein intelligentes Anästhesiesystem mit prädiktiver Analytik, das die Präzisionsdosierung in chirurgischen Umgebungen in Deutschland und Frankreich verbessern soll.
- Im Oktober 2023 brachte die Drägerwerk AG eine neue Reihe kompakter Beatmungsgeräte auf den Markt, die speziell für den ambulanten und transportablen Einsatz entwickelt wurden und damit der wachsenden Nachfrage nach Notfall- und mobiler Beatmungsversorgung in Großbritannien und den nordischen Ländern gerecht werden.
- Im August 2023 kündigte die Hamilton Medical AG die Einführung einer Cloud-fähigen Beatmungsmanagement-Plattform in ganz Europa an, die die Fernüberwachungsmöglichkeiten auf Intensivstationen verbessert.
- Im Juni 2023 ging Vyaire Medical eine Partnerschaft mit führenden Krankenhäusern in Italien und Spanien ein, um sein automatisiertes Entwöhnungssystem für die mechanische Beatmung zu testen. Ziel ist es, die Aufenthalte auf der Intensivstation zu verkürzen und die Genesungszeiten der Patienten zu verbessern.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.