Europäischer Adalimumab-Markt, nach Indikation ( rheumatoide Arthritis , juvenile idiopathische Arthritis, Psoriasis-Arthritis, ankylosierende Spondylitis, Morbus Crohn, Hidradenitis suppurativa, Colitis ulcerosa, chronische Plaque-Psoriasis, nicht infektiöse Intermediärpräparate und andere), Typ (Biologika und Biosimilars), Dosierungsstärke (20 mg/0,4 ml/g, 40 mg/0,8 ml/g und andere), Arzneimitteltyp (Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio, Idacio), Bevölkerungstyp (Kinder und Erwachsene), Endverbraucher (Krankenhäuser, Fachkliniken, häusliche Pflege und andere), Vertriebskanal (Krankenhausapotheken, Einzelhandelsapotheken, Online-Apotheken und andere), Branchentrends und Prognose bis 2029.
Marktdefinition
Adalimumab ist einer der monoklonalen Antikörper, die zur Behandlung bestimmter Autoimmunerkrankungen wie rheumatoide Arthritis und Morbus Crohn eingesetzt werden. Adalimumab ist ein Anti-TNF-Medikament, das zur Behandlung entzündlicher Symptome eingesetzt wird. Das biologische Präparat von Adalimumab ist Humira, und es sind auch verschiedene Biosimilar-Medikamente von Humira erhältlich, darunter Exemptia, Hyrimoz, Cyltezo und Hulio. Adalimumab wirkt, indem es sich an den TNF-Faktor Alpha bindet, wodurch die Wahrscheinlichkeit einer entzündlichen Reaktion auf Autoimmunerkrankungen verringert wird.
Marktanalyse und Einblicke
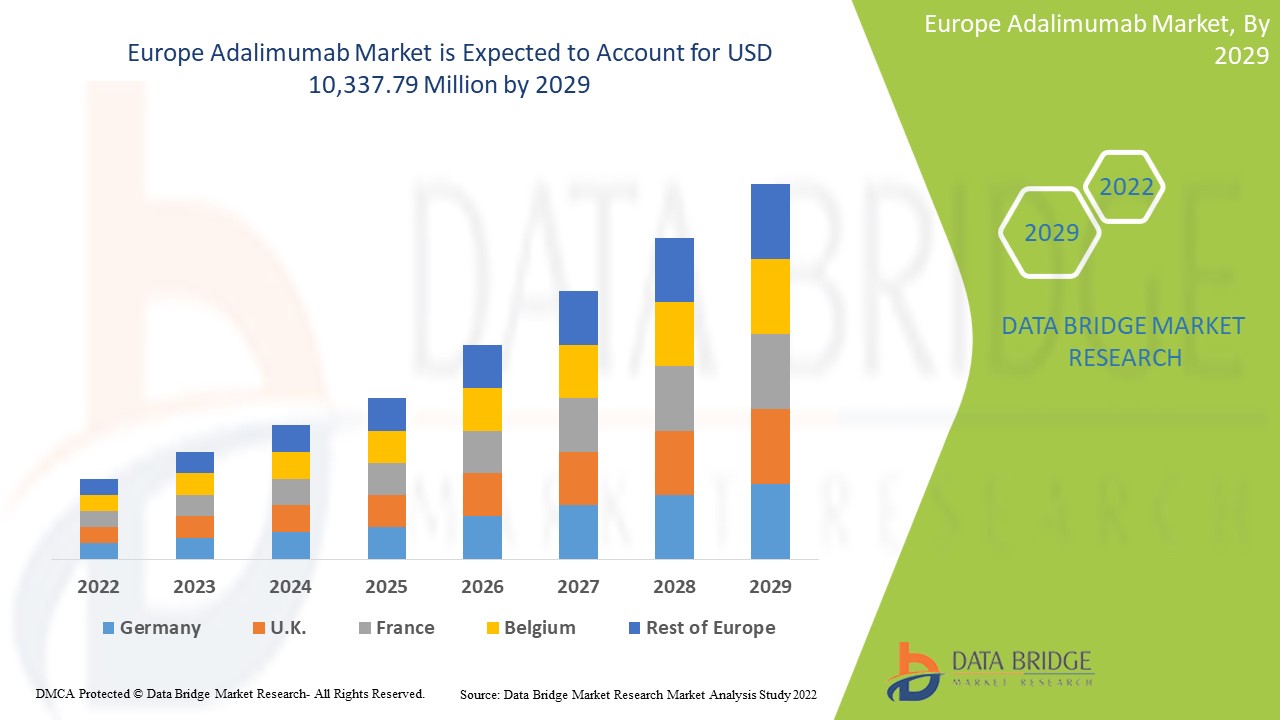
Der europäische Adalimumab-Markt wird im Prognosezeitraum 2022 bis 2029 voraussichtlich ein Marktwachstum verzeichnen. Data Bridge Market Research analysiert, dass der Markt im Prognosezeitraum 2022 bis 2029 mit einer CAGR von 16,2 % wächst und von 3.485,94 Millionen USD im Jahr 2021 auf 10.337,79 Millionen USD im Jahr 2029 ansteigen dürfte.
|
Berichtsmetrik |
Details |
|
Prognosezeitraum |
2022 bis 2029 |
|
Basisjahr |
2021 |
|
Historische Jahre |
2020 |
|
Quantitative Einheiten |
Umsatz in Mio. USD |
|
Abgedeckte Segmente |
Nach Indikation (Rheumatoide Arthritis, Juvenile idiopathische Arthritis, Psoriasis-Arthritis, Spondylitis ankylosans, Morbus Crohn , Hidradenitis suppurativa, Colitis ulcerosa, chronische Plaque-Psoriasis, nicht infektiöse Intermediärform und andere), Typ (Biologika und Biosimilars) , Dosierungsstärke (20 mg/0,4 ml/g, 40 mg/0,8 ml/g und andere), Arzneimitteltyp (Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio, Idacio), Populationstyp (Kinder und Erwachsene), Endverbraucher (Krankenhäuser, Fachkliniken, häusliche Pflege und andere), Vertriebskanal (Krankenhausapotheken, Einzelhandelsapotheken, Online-Apotheken und andere) |
|
Abgedeckte Länder |
Deutschland, Frankreich, Italien, Großbritannien, Spanien, Niederlande, Russland, Schweiz, Belgien, Türkei, Ungarn, Litauen, Österreich, Irland, Norwegen, Polen und Rest von Europa |
|
Abgedeckte Marktteilnehmer |
Die wichtigsten Unternehmen, die auf dem Markt tätig sind, sind unter anderem AbbVie Inc., Sandoz International GmbH, Amgen Inc., Mylan NV (eine Tochtergesellschaft von Viatris), Biogen, Celltrion Healthcare Co., Ltd., Fresenius Kabi SwissBioSim GmbH, Alvotech, Biocad, Coherus BioSciences, Shanghai Henlius Biotech, Inc., Synermore Biologics, Prestige BioPharma Ltd. und Janssen Global Services, LLC. |
Adalimumab Marktdynamik
Treiber
- Zunehmende Prävalenz der rheumatoiden Arthritis
Die Prävalenz der rheumatoiden Arthritis nimmt weltweit zu. Es wurde berichtet, dass die jährliche Inzidenz von RA weltweit bei fast 3 Fällen pro 10.000 Einwohner liegt. Rheumatoide Arthritis führt zur Entwicklung entzündlicher Symptome, die mit verschiedenen Arzneimitteltherapien, darunter auch biologischen Therapien, behandelt werden können. Eine der innovativsten biologischen Therapien zur Behandlung von rheumatoider Arthritis ist Adalimumab, ein monoklonaler Antikörper, der die Immunzellen angreift und die Rekrutierung von Immunzellen verlangsamt, was zu einer Verringerung der Entzündung an der Zielstelle führt. Der kontinuierliche Anstieg der rheumatoiden Arthritis weltweit und in der europäischen Region hat das Leben der Betroffenen stark beeinträchtigt und daher stellt die Krankheit auch eine große Belastung für das medizinische Fachpersonal dar.
- Anstieg der geriatrischen Bevölkerung
Die Zahl der geriatrischen Menschen nimmt zu und die Menschen leben seit längerem mit verschiedenen Arten von chronischen Krankheiten. Es wurde berichtet, dass die Alterung der Bevölkerung zu schnell voranschreitet und sich dramatisch ausbreitet. Die Erkrankung rheumatoide Arthritis soll mit der Zunahme der älteren Bevölkerung in Zusammenhang stehen. Einer Studie zufolge waren im Jahr 2019 etwa 703 Millionen Menschen über 65 Jahre alt. In Nordamerika und Europa gab es über 200 Millionen ältere Menschen. Die Studie deutete auch darauf hin, dass die Bevölkerung bereits deutlich älter ist und langsam um etwa 48 % ansteigt. Die Zahl der Erwachsenen, die an rheumatoider Arthritis leiden, nimmt zu und ist weitgehend auf eine Adalimumab-Therapie angewiesen. Dies bedeutet, dass die zunehmende geriatrische Bevölkerung als Treiber für das Marktwachstum fungiert.
- Zunehmende Anzahl von Auftragsforschungsinstituten (CROs)
Auftragsforschungsinstitute unterstützen klinische Studien und andere Forschungsaktivitäten für Medikamente und medizinische Geräte. Auftragsforschungsinstitute (CROs) unterstützen die Biotechnologie- und Pharmaindustrie bei der Arzneimittelentwicklung und senken die Gesamtkosten klinischer Studien. Da verschiedene Adalimumab-Medikamente derzeit intensiv in klinischen Studien getestet werden, um den unerfüllten Bedarf von Patienten mit entzündlichen Erkrankungen zu decken, fördert die steigende Zahl von Auftragsforschungsinstituten das Marktwachstum. Die Zahl der Auftragsforschungsinstitute nimmt zu, was den Prozess der Entwicklung von Adalimumab-Medikamenten vereinfacht und die für die Arzneimittelentwicklung benötigte Zeit verkürzt hat.
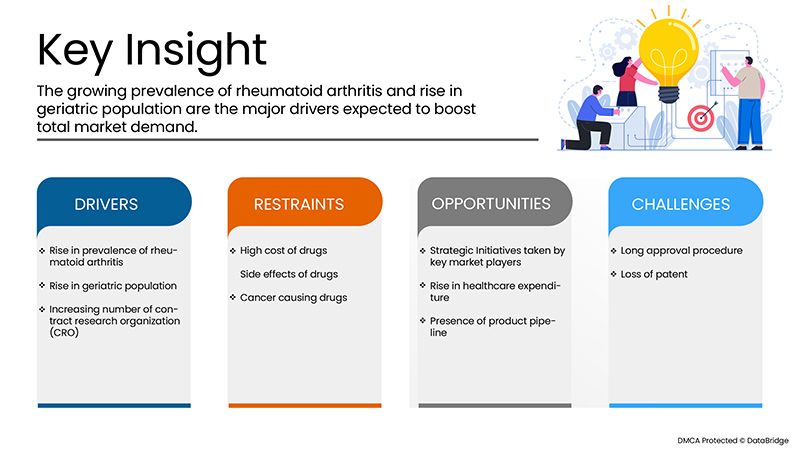
Gelegenheiten
- Strategische Initiativen der Marktteilnehmer
Die Nachfrage nach Adalimumab ist sowohl in der Region als auch weltweit gestiegen, da es immer mehr chronische Krankheiten gibt und die Zahl der geriatrischen Menschen zunimmt. Diese günstigen Faktoren steigern den Bedarf an den Medikamenten, und um die Marktnachfrage zu decken, nutzen kleine und große Marktteilnehmer verschiedene Strategien.
Darüber hinaus wird der Anstieg der staatlichen Gesundheitsausgaben in der Region dem Adalimumab-Markt im Prognosezeitraum 2022–2029 strukturelle Integrität und Zukunftschancen verleihen.
Einschränkungen/Herausforderungen
Die hohen Kosten der Medikamente und einige Nebenwirkungen, die nach der Einnahme der Medikamente auftreten, können jedoch das Wachstum des Marktes behindern. Darüber hinaus werden strenge Regeln und Vorschriften den Markt im oben genannten Prognosezeitraum vor weitere Herausforderungen stellen.
Dieser Adalimumab-Marktbericht enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-Export-Analysen, Produktionsanalysen, Wertschöpfungskettenoptimierungen, Marktanteilen, Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neue Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um weitere Informationen zum Arthritis-Markt zu erhalten, wenden Sie sich an Data Bridge Market Research, um einen Analystenbericht zu erhalten. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Auswirkungen von COVID-19 auf den Adalimumab-Markt
COVID-19 hat das Marktwachstum nicht so stark beeinflusst. Da die Prävalenz verschiedener Krankheiten in der Region wie rheumatoide Arthritis, Morbus Crohn und andere zunimmt, führt dies zu einer Zunahme der Entwicklung verschiedener Medikamente auf dem Markt. Daher steigt auch in der COVID-19-Periode der Bedarf an Adalimumab-Medikamenten weiter an.
Jüngste Entwicklung
- Im Oktober 2018 gab Sandoz, eine Division von Novartis und Pionier und weltweit führender Anbieter von Biosimilars, heute bekannt, dass die US-amerikanische Food and Drug Administration (FDA) ihr Biosimilar HyrimozTM (Adalimumab-adaz) zugelassen hat. Die FDA erteilte die Zulassung für die Behandlung von rheumatoider Arthritis (RA), juveniler idiopathischer Arthritis (JIA) bei Patienten ab vier Jahren, Psoriasis-Arthritis (PsA), ankylosierender Spondylitis (AS), Morbus Crohn (CD) bei Erwachsenen, Colitis ulcerosa (UC) und Plaque-Psoriasis (Ps).
Adalimumab-Marktumfang in Europa
Der europäische Adalimumab-Markt ist nach Indikation, Typ, Dosierungsstärke, Arzneimitteltyp, Bevölkerungstyp, Endverbraucher und Vertriebskanal segmentiert. Das Wachstum in diesen Segmenten hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, um strategische Entscheidungen zur Identifizierung der wichtigsten Marktanwendungen zu treffen.
Anzeige
- Rheumatoide Arthritis
- Juvenile idiopathische Arthritis
- Psoriasis-Arthritis
- Spondylitis ankylosans
- Morbus Crohn
- Hidradenitis suppurativa (verstärkte Hidradenitis)
- Colitis ulcerosa
- Chronische Plaque-Psoriasis
- Nichtinfektiöses Mittel
- Sonstiges
Auf der Grundlage der Indikation ist der europäische Adalimumab-Markt in rheumatoide Arthritis, juvenile idiopathische Arthritis, Psoriasis-Arthritis, ankylosierende Spondylitis, Morbus Crohn, Hidradenitis suppurativa, Colitis ulcerosa, chronische Plaque-Psoriasis, nicht infektiöse intermediäre Formen und andere unterteilt.
Typ
- Biologika
- Biosimilars
Auf der Grundlage des Typs ist der europäische Adalimumab-Markt in Biologika und Biosimilars segmentiert.
Dosierungsstärke
- 20 MG/0,4 MLG
- 40 mg/0,8 mg/kg
- Sonstiges
Auf der Grundlage der Dosierungsstärke ist der europäische Adalimumab-Markt in 20 mg/0,4 mlg, 40 mg/0,8 mlg und andere unterteilt.
Arzneimitteltyp
- Humira
- Amgevita
- Imraldi
- Hyrimoz
- Abonnieren
- Hulio
- Idacio
Auf der Grundlage der Arzneimittelart ist der europäische Adalimumab-Markt in Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio und Idacio unterteilt.
Bevölkerungstyp
- Kinder
- Erwachsene
Auf der Grundlage der Bevölkerungsgruppe ist der europäische Adalimumab-Markt in Kinder und Erwachsene segmentiert.
Endbenutzer
- Krankenhäuser
- Spezialkliniken
- Häusliche Gesundheitspflege
- Sonstiges
Auf der Grundlage der Endverbraucher ist der europäische Adalimumab-Markt in Krankenhäuser, Fachkliniken, häusliche Pflege und Sonstige unterteilt.
Vertriebskanal
- Krankenhausapotheken
- Einzelhandelsapotheken
- Online-Apotheken
- Sonstiges
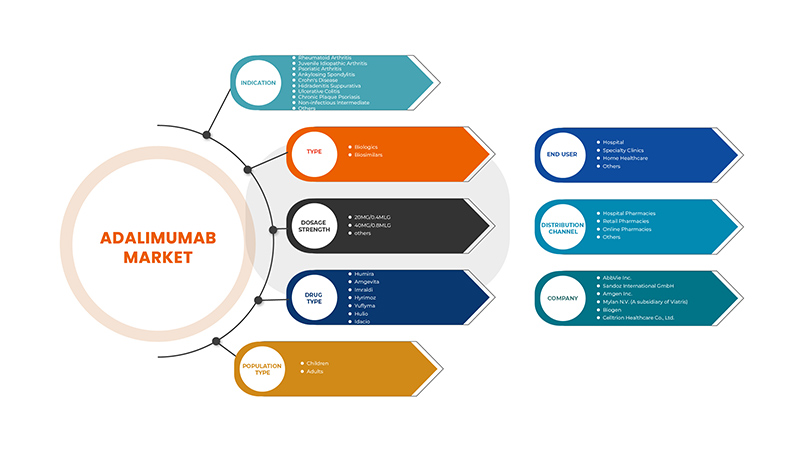
Auf der Grundlage der Vertriebskanäle ist der europäische Adalimumab-Markt in Krankenhausapotheken, Einzelhandelsapotheken, Online-Apotheken und andere unterteilt.
Adalimumab Markt – Regionale Analyse/Einblicke
Der Adalimumab-Markt wird analysiert und es werden Einblicke in die Marktgröße und Trends nach Land, Indikation, Typ, Dosierungsstärke, Arzneimitteltyp, Bevölkerungstyp, Endverbraucher und Vertriebskanal wie oben angegeben bereitgestellt.
Der europäische Adalimumab-Markt ist weiter in folgende Länder unterteilt: Deutschland, Frankreich, Italien, Großbritannien, Spanien, Niederlande, Russland, Schweiz, Belgien, Türkei, Ungarn, Litauen, Österreich, Irland, Norwegen, Polen und Rest des Europas.
Deutschland dominiert den Adalimumab-Markt in Bezug auf Marktanteil und Marktumsatz und wird seine Dominanz im Prognosezeitraum weiter ausbauen. Dies ist auf die zunehmende Verbreitung verschiedener chronischer Krankheiten zurückzuführen, und Forschung und Entwicklung in der Arzneimittelentwicklung in der Region Deutschland fördern dieses Marktwachstum weiter.
Der Länderabschnitt des Berichts enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Marktvorschriften, die sich auf die aktuellen und zukünftigen Markttrends auswirken. Datenpunkte wie Neu- und Ersatzverkäufe, demografische Daten des Landes, Krankheitsepidemiologie und Import- und Exportzölle sind einige der wichtigsten Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Darüber hinaus werden bei der Prognoseanalyse der Länderdaten die Präsenz und Verfügbarkeit globaler Marken und ihre Herausforderungen aufgrund der hohen Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen der Vertriebskanäle berücksichtigt.
Wettbewerbsumfeld und Marktanteilsanalyse
Die Wettbewerbslandschaft des Adalimumab-Marktes liefert Einzelheiten zu den Wettbewerbern. Zu den enthaltenen Details gehören Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen auf den Adalimumab-Markt.
Zu den wichtigsten Akteuren auf dem Adalimumab-Markt zählen unter anderem AbbVie Inc., Sandoz International GmbH, Amgen Inc., Mylan NV (eine Tochtergesellschaft von Viatris), Biogen, Celltrion Healthcare Co., Ltd., Fresenius Kabi SwissBioSim GmbH, Alvotech, Biocad, Coherus BioSciences, Shanghai Henlius Biotech, Inc., Synermore Biologics, Prestige BioPharma Ltd. und Janssen Global Services, LLC.
Forschungsmethodik
Die Datenerfassung und die Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Die wichtigste Forschungsmethode, die das DBMR-Forschungsteam verwendet, ist die Datentriangulation, die Data Mining, Analyse der Auswirkungen von Datenvariablen auf den Markt und primäre (Branchenexperten-)Validierung umfasst. Darüber hinaus umfassen die Datenmodelle ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, einen Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Firmenmarktanteilsanalyse, Messstandards, Europa vs. Region und Lieferantenanteilsanalyse. Bitte fordern Sie bei weiteren Fragen einen Analystenanruf an.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen im Bereich der fortschrittlichen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersichten, Analysen des Marktes für aufgearbeitete Produkte und der Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien durchgeführt werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten benötigen, und zwar in dem von Ihnen gewünschten Format und Datenstil. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien oder Pivot-Tabellen (Factbook) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE ADALIMUMAB MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 INDICATION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PIPELINE ANALYSIS
4 REGULATORY FRAMEWORK OF EUROPE ADALIMUMAB MARKET
5 EPIDEMIOLOGY
6 ADALIMUMAB PRESCRIPTION
7 EUROPE ADALIMUMAB MARKET: REIMBURSEMENT SCENARIO
7.1 REIMBURSEMENT SCENARIO IN THE U.S.
7.2 REIMBURSEMENT SCENARIO IN CHINA
7.3 REIMBURSEMENT SCENARIO IN JAPAN
7.4 REIMBURSEMENT IN CENTRAL AND EASTERN EUROPE
7.5 REIMBURSEMENT SCENARIO IN DENMARK
7.6 REIMBURSEMENT SCENARIO IN IRELAND
8 IMPACT OF BIOSIMILAR
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN THE PREVALENCE OF RHEUMATOID ARHTRITIS
9.1.2 INCREASING GERIATRIC POPULATION
9.1.3 INCREASING NUMBER OF CONTRACT RESEARCH ORGANIZATIONS
9.1.4 INTRODUCTION TO BIOSIMILARS
9.1.5 EXPLORATION OF EMERGING MARKETS
9.2 RESTRAINTS
9.2.1 HIGH COSTS OF DRUGS
9.2.2 SIDE EFFECTS OF DRUGS
9.2.3 CANCER CAUSING DRUGS
9.3 OPPORTUNITIES
9.3.1 PRESENCE OF PRODUCT PIPELINE
9.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.3 INCREASING HEALTHCARE EXPENDITURE
9.3.4 PRESENCE OF REIMBURSEMENT POLICIES
9.4 CHALLENGES
9.4.1 LOSS OF PATENTS
9.4.2 AVAILABILITY OF ALTERNATIVES
9.4.3 LONG APPROVAL PROCEDURE
10 COVID-19 IMPACT ON ADALIMUMAB IN HEALTHCARE INDUSTRY
10.1 OVERVIEW
10.2 ADALIMUMAB AND COVID-19
10.3 PRICE IMPACT OF COVID-19
10.4 IMPACT ON DEMAND
10.5 IMPACT ON SUPPLY CHAIN
10.6 STRATEGIC DECISIONS FOR MANUFACTURERS
10.7 CONCLUSION
11 EUROPE ADALIMUMAB MARKET, BY INDICATION
11.1 OVERVIEW
11.2 RHEUMATOID ARTHRITIS
11.3 ANKYLOSING SPONDYLITIS
11.4 CHRONIC PLAQUE PSORIASIS
11.5 CROHN’S DISEASE
11.6 ULCERATIVE COLITIS
11.7 PSORIATIC ARTHRITIS
11.8 JUVENILE IDIOPATHIC ARTHRITIS
11.9 HIDRADENITIS SUPPURATIVA
11.1 NON-INFECTIOUS INTERMEDIATE
11.11 OTHERS
12 EUROPE ADALIMUMAB MARKET, BY TYPE
12.1 OVERVIEW
12.2 BIOLOGICS
12.3 BIOSIMILARS
12.3.1 ADALIMUMAB-ATTO
12.3.2 ADALIMUMAB-BWWD
12.3.3 ADALIMUMAB-ADBM
12.3.4 ADALIMUMAB-ADAZ
12.3.5 ADALIMUMAB-FKJP
12.3.6 ADALIMUMAB-AFZB
12.3.7 OTHERS
13 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGTH
13.1 OVERVIEW
13.2MG/0.4ML
13.3MG/0.8ML
13.4MG/0.4ML
13.5MG/0.1ML
13.6 OTHERS
14 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
14.3.1 AMJEVITA
14.3.2 HYRIMOZ
14.3.3 HULIO
14.3.4 OTHERS
15 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 PARENTERAL
15.3 ORAL
16 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE
16.1 OVERVIEW
16.2 ADULTS
16.3 CHILDREN
17 EUROPE ADALIMUMAB MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 DIRECT TENDER
18.6 OTHERS
19 EUROPE ADALIMUMAB MARKET, BY GEOGRAPHY
19.1 EUROPE
19.1.1 GERMANY
19.1.2 U.K
19.1.3 ITALY
19.1.4 FRANCE
19.1.5 SPAIN
19.1.6 NETHERLANDS
19.1.7 RUSSIA
19.1.8 SWITZERLAND
19.1.9 BELGIUM
19.1.10 TURKEY
19.1.11 AUSTRIA
19.1.12 NORWAY
19.1.13 HUNGARY
19.1.14 LITHUANIA
19.1.15 IRELAND
19.1.16 POLAND
19.1.17 REST OF EUROPE
20 EUROPE ADALIMUMAB MARKET: COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: EUROPE
21 SWOT
22 COMPANY PROFILES
22.1 ABBVIE INC.
22.1.1 COMPANY SNAPSHOT
22.1.2 REVENUE ANALYSIS
22.1.3 COMPANY SHARE ANALYSIS
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 AMGEN (EUROPE) GMBH (A SUBSIDIARY OF AMGEN INC.)
22.2.1 COMPANY SNAPSHOT
22.2.2 REVENUE ANALYSIS
22.2.3 COMPANY SHARE ANALYSIS
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BIOGEN
22.3.1 COMPANY SNAPSHOT
22.3.2 REVENUE ANALYSIS
22.3.3 PRODUCT PORTFOLIO
22.3.4 RECENT DEVELOPMENTS
22.4 SANDOZ INTERNATIONAL GMBH {A SUBSIDIARY OF SANDOZ (A DIVISION OF NOVARTIS AG)}
22.4.1 COMPANY SNAPSHOT
22.4.2 REVENUE ANALYSIS
22.4.3 PRODUCT PORTFOLIO
22.4.4 RECENT DEVELOPMENTS
22.5 MYLAN N.V.
22.5.1 COMPANY SNAPSHOT
22.5.2 REVENUE ANALYSIS
22.5.3 PRODUCT PORTFOLIO
22.5.4 RECENT DEVELOPMENTS
22.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
22.6.1 COMPANY SNAPSHOT
22.6.2 REVENUE ANALYSIS
22.6.3 PRODUCT PORTFOLIO
22.6.4 RECENT DEVELOPMENTS
22.7 CELLTRION INC.
22.7.1 COMPANY SNAPSHOT
22.7.2 REVENUE ANALYSIS
22.7.3 PRODUCT PORTFOLIO
22.7.4 RECENT DEVELOPMENTS
22.8 COHERUS BIOSCIENCES
22.8.1 COMPANY SNAPSHOT
22.8.2 PRODUCT PORTFOLIO
22.8.3 RECENT DEVELOPMENTS
22.9 FRESENIUS KABI DEUTSCHLAND GMBH (A SUBSIDIARY OF FRESENIUS KABI AG)
22.9.1 COMPANY SNAPSHOT
22.9.2 REVENUE ANALYSIS
22.9.3 PRODUCT PORTFOLIO
22.9.4 RECENT DEVELOPMENTS
22.1 HETERO BIOPHARMA LTD.
22.10.1 COMPANY SNAPSHOT
22.10.2 PRODUCT PORTFOLIO
22.10.3 RECENT DEVELOPMENTS
22.11 INNOVENT BIOLOGICS, INC.
22.11.1 COMPANY SNAPSHOT
22.11.2 REVENUE ANALYSIS
22.11.3 PRODUCT PORTFOLIO
22.11.4 RECENT DEVELOPMENTS
22.12 PFIZER INC.
22.12.1 COMPANY SNAPSHOT
22.12.2 REVENUE ANALYSIS
22.12.3 PRODUCT PORTFOLIO
22.12.4 RECENT DEVELOPMENTS
22.13 RELIANCE LIFE SCIENCES (A SUBSIDIARY OF RELIANCE INDUSTRIES LIMITED)
22.13.1 COMPANY SNAPSHOT
22.13.2 REVENUE ANALYSIS
22.13.3 PRODUCT PORTFOLIO
22.13.4 RECENT DEVELOPMENTS
22.14 SAMSUNG BIOEPIS (A SUBSIDIARY OF SAMSUNG BIOLOGICS)
22.14.1 COMPANY SNAPSHOT
22.14.2 REVENUE ANALYSIS
22.14.3 PRODUCT PORTFOLIO
22.14.4 RECENT DEVELOPMENTS
22.15 ZYDUS CADILA
22.15.1 COMPANY SNAPSHOT
22.15.2 REVENUE ANALYSIS
22.15.3 PRODUCT PORTFOLIO
22.15.4 RECENT DEVELOPMENT
23 QUESTIONNAIRE
24 RELATED REPORTS
Tabellenverzeichnis
LIST OF TABLES
TABLE 1 EUROPE ADALIMUMAB MARKET, PIPELINE ANALYSIS
TABLE 2 BIOSIMILAR OF ADALIMUMAB LAUNCHED IN THE U.S.
TABLE 3 PREVALENCE AND INCIDENCE RATES OF RA WORLDWIDE (CASE PER 100 INHABITANTS)
TABLE 4 BIOLOGIC DRUGS SUBJECTED TO PATENT LOSS
TABLE 5 ALTERNATIVE DRUGS FOR INFLAMMATORY DISEASES TREATMENT
TABLE 6 EUROPE ADALIMUMAB MARKET, BY INDICATION 2019-2027 (USD MILLION)
TABLE 7 EUROPE RHEUMATOID ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 8 EUROPE ANKYLOSING SPONDYLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 9 EUROPE CHRONIC PLAQUE PSORIASIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 10 EUROPE CROHN’S DISEASE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 11 EUROPE ULCERATIVE COLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 12 EUROPE PSORIATIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 13 EUROPE JUVENILE IDIOPATHIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 14 EUROPE HIDRADENITIS SUPPURATIVA IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 15 EUROPE NONINFECTIOUS INTERMEDIATE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 16 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 17 EUROPE ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 18 EUROPE BIOLOGICS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 19 EUROPE BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 20 EUROPE BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 21 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGHT, 2019-2027 (USD MILLION)
TABLE 22 EUROPE 40MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 23 EUROPE 80MG/0.8ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 24 EUROPE 20MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 25 EUROPE 10MG/0.1ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 26 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 27 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE, 2019-2027 (USD MILLION)
TABLE 28 EUROPE BRANDED IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 29 EUROPE GENERICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 30 EUROPE GENERICS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 31 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION 2019-2027 (USD MILLION)
TABLE 32 EUROPE PARENTERAL IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 33 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE, 2019-2027 (USD MILLION)
TABLE 34 EUROPE ADULTS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 35 EUROPE CHILDREN IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 36 EUROPE ADALIMUMAB MARKET, BY END USER, 2019-2027 (USD MILLION)
TABLE 37 EUROPE HOSPITALS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 38 EUROPE SPECIALTY CLINICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 39 EUROPE HOME HEALTHCARE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 40 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 41 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
TABLE 42 EUROPE HOSPITAL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 43 EUROPE RETAIL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 44 EUROPE ONLINE PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 45 EUROPE DIRECT TENDER IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 46 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 47 EUROPE ADALIMUMAB MARKET, BY COUNTRY, 2018-2027 (USD MILLION)
TABLE 48 EUROPE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 49 EUROPE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 50 EUROPE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 51 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 52 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 53 EUROPE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 54 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 55 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 56 EUROPE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 57 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 58 GERMANYADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 59 GERMANYADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 60 GERMANYBIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 61 GERMANYADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 62 GERMANYADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 63 GERMANY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 64 GERMANYADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 65 GERMANYADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 66 GERMANYADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 67 GERMANYADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 68 U.K ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 69 U.K ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 70 U.K BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 71 U.K ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 72 U.K ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 73 U.K GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 74 U.K ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 75 U.K ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 76 U.K ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 77 U.K ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 78 ITALY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 79 ITALY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 80 ITALY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 81 ITALY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 82 ITALY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 83 ITALY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 84 ITALY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 85 ITALY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 86 ITALY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 87 ITALY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 88 FRANCE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 89 FRANCE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 90 FRANCE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 91 FRANCE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 92 FRANCE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 93 FRANCE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 94 FRANCE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 95 FRANCE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 96 FRANCE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 97 FRANCE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 98 SPAIN ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 99 SPAIN ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 100 SPAIN BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 101 SPAIN ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 102 SPAIN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 103 SPAIN GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 104 SPAIN ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 105 SPAIN ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 106 SPAIN ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 107 SPAIN ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 108 NETHERLANDS ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 109 NETHERLANDS ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 110 NETHERLANDS BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 111 NETHERLANDS ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 112 NETHERLANDS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 113 NETHERLANDS GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 114 NETHERLANDS ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 115 NETHERLANDS ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 116 NETHERLANDS ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 117 NETHERLANDS ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 118 RUSSIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 119 RUSSIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 120 RUSSIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 121 RUSSIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 122 RUSSIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 123 RUSSIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 124 RUSSIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 125 RUSSIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 126 RUSSIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 127 RUSSIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 128 SWITZERLAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 129 SWITZERLAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 130 SWITZERLAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 131 SWITZERLAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 132 SWITZERLAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 133 SWITZERLAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 134 SWITZERLAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 135 SWITZERLAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 136 SWITZERLAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 137 SWITZERLAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 138 BELGIUM ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 139 BELGIUM ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 140 BELGIUM BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 141 BELGIUM ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 142 BELGIUM ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 143 BELGIUM GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 144 BELGIUM ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 145 BELGIUM ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 146 BELGIUM ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 147 BELGIUM ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 148 TURKEY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 149 TURKEY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 150 TURKEY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 151 TURKEY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 152 TURKEY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 153 TURKEY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 154 TURKEY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 155 TURKEY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 156 TURKEY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 157 TURKEY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 158 AUSTRIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 159 AUSTRIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 160 AUSTRIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 161 AUSTRIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 162 AUSTRIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 163 AUSTRIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 164 AUSTRIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 165 AUSTRIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 166 AUSTRIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 167 AUSTRIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 168 NORWAY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 169 NORWAY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 170 NORWAY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 171 NORWAY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 172 NORWAY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 173 NORWAY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 174 NORWAY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 175 NORWAY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 176 NORWAY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 177 NORWAY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 178 HUNGARY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 179 HUNGARY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 180 HUNGARY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 181 HUNGARY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 182 HUNGARY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 183 HUNGARY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 184 HUNGARY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 185 HUNGARY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 186 HUNGARY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 187 HUNGARY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 188 LITHUANIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 189 LITHUANIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 190 LITHUANIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 191 LITHUANIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 192 LITHUANIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 193 LITHUANIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 194 LITHUANIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 195 LITHUANIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 196 LITHUANIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 197 LITHUANIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 198 IRELAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 199 IRELAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 200 IRELAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 201 IRELAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 202 IRELAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 203 IRELAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 204 IRELAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 205 IRELAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 206 IRELAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 207 IRELAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 208 POLAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 209 POLAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 210 POLAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 211 POLAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 212 POLAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 213 POLAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 214 POLAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 215 POLAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 216 POLAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 217 POLAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 218 REST OF EUROPE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
Abbildungsverzeichnis
LIST OF FIGURES
FIGURE 1 EUROPE ADALIMUMAB MARKET: SEGMENTATION
FIGURE 2 EUROPE ADALIMUMAB MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE ADALIMUMAB MARKET: DROC ANALYSIS
FIGURE 4 EUROPE ADALIMUMAB MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE ADALIMUMAB MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE ADALIMUMAB MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE ADALIMUMAB MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE ADALIMUMAB MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE ADALIMUMAB MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE ADALIMUMAB MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF RHEUMATOID ARTHRITIS AND INCREASING GERIATRIC POPULATION IS DRIVING THE EUROPE ADALIMUMAB MARKET IN THE FORECAST PERIOD OF 2020 TO 2027
FIGURE 12 RHEUMATOID ARTHRITIS IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ADALIMUMAB MARKET IN 2020 & 2027
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE ADALIMUMAB MARKET
FIGURE 14 MARKET GROWTH IN CLINICAL CRO (IN USD MILLIONS)
FIGURE 15 FUNCTION OF CRO
FIGURE 16 HEALTHCARE EXPENDITURE IN 2016 AND 2019
FIGURE 17 EUROPE ADALIMUMAB MARKET: BY INDICATION, 2019
FIGURE 18 EUROPE ADALIMUMAB MARKET: BY INDICATION, 2019-2027 (USD MILLION)
FIGURE 19 EUROPE ADALIMUMAB MARKET: BY INDICATION, CAGR (2020-2027)
FIGURE 20 EUROPE ADALIMUMAB MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 21 EUROPE ADALIMUMAB MARKET: BY TYPE, 2019
FIGURE 22 EUROPE ADALIMUMAB MARKET: BY TYPE 2019-2027 (USD MILLION)
FIGURE 23 EUROPE ADALIMUMAB MARKET: BY TYPE, CAGR (2020-2027)
FIGURE 24 EUROPE ADALIMUMAB MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, 2019
FIGURE 26 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH 2019-2027 (USD MILLION)
FIGURE 27 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, CAGR (2020-2027)
FIGURE 28 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, LIFELINE CURVE
FIGURE 29 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, 2019
FIGURE 30 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE , 2019-2027 (USD MILLION)
FIGURE 31 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, CAGR (2020-2027)
FIGURE 32 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 33 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019
FIGURE 34 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019-2027 (USD MILLION)
FIGURE 35 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2020-2027)
FIGURE 36 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 37 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, 2019
FIGURE 38 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, 2019-2027 (USD MILLION)
FIGURE 39 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, CAGR (2020-2027)
FIGURE 40 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 41 EUROPE ADALIMUMAB MARKET: BY END USER, 2019
FIGURE 42 EUROPE ADALIMUMAB MARKET: BY END USER, 2019-2027 (USD MILLION)
FIGURE 43 EUROPE ADALIMUMAB MARKET: BY END USER, CAGR (2020-2027)
FIGURE 44 EUROPE ADALIMUMAB MARKET: BY END USER, LIFELINE CURVE
FIGURE 45 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019
FIGURE 46 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
FIGURE 47 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, CAGR (2020-2027)
FIGURE 48 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 49 EUROPE ADALIMUMAB MARKET: SNAPSHOT (2019)
FIGURE 50 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2019)
FIGURE 51 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2020 & 2027)
FIGURE 52 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2019 & 2027)
FIGURE 53 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE (2020-2027)
FIGURE 54 EUROPE ADALIMUMAB MARKET: COMPANY SHARE 2019 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.