Asia Pacific Vaccines Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
9,048.51 Million
USD
18,031.69 Million
2022
2030
USD
9,048.51 Million
USD
18,031.69 Million
2022
2030
| 2023 –2030 | |
| USD 9,048.51 Million | |
| USD 18,031.69 Million | |
|
|
|
Impfstoffmarkt im asiatisch-pazifischen Raum nach Zusammensetzung (Kombinationsimpfstoffe und Monoimpfstoffe), Typ (Untereinheiten-, rekombinante, Polysaccharid- und Konjugatimpfstoffe, Lebendimpfstoffe, inaktivierte Impfstoffe und Toxoidimpfstoffe), Art (Routineimpfstoff, empfohlener Impfstoff und vorgeschriebener Impfstoff), Verabreichungsalter (Kinderimpfstoff und Erwachsenenimpfstoff), Krankheiten (Pneumokokken-Erkrankung, Masern, Mumps und Windpocken, DPT, Hepatitis, Grippe, Typhus, Meningokokken, Tollwut, Japanische Enzephalitis, Gelbfieber und andere), Verabreichungsweg (injizierbar, oral und nasal), Endverbraucher (Gemeindekrankenhäuser, Krankenhäuser, Fachzentren, Kliniken und andere), Vertriebskanal (Krankenhausapotheke, Einzelhandelsapotheke und Online-Apotheke) – Branchentrends und Prognose bis 2030.
Marktanalyse und Einblicke für Impfstoffe im asiatisch-pazifischen Raum
Die zunehmende Verbreitung von Infektionskrankheiten, darunter bakterielle und virale Erkrankungen, beschert dem Markt lukratives Wachstum. Darüber hinaus kurbeln auch die zunehmende staatliche Unterstützung und die Einführung neuerer Impfstoffe den Impfstoffmarkt an. Ein weiterer Faktor, der das Wachstum des Impfstoffmarktes ankurbelt, ist das zunehmende Bewusstsein für Impfungen und die Nachfrage nach wirksamen COVID-19-Impfstoffen.
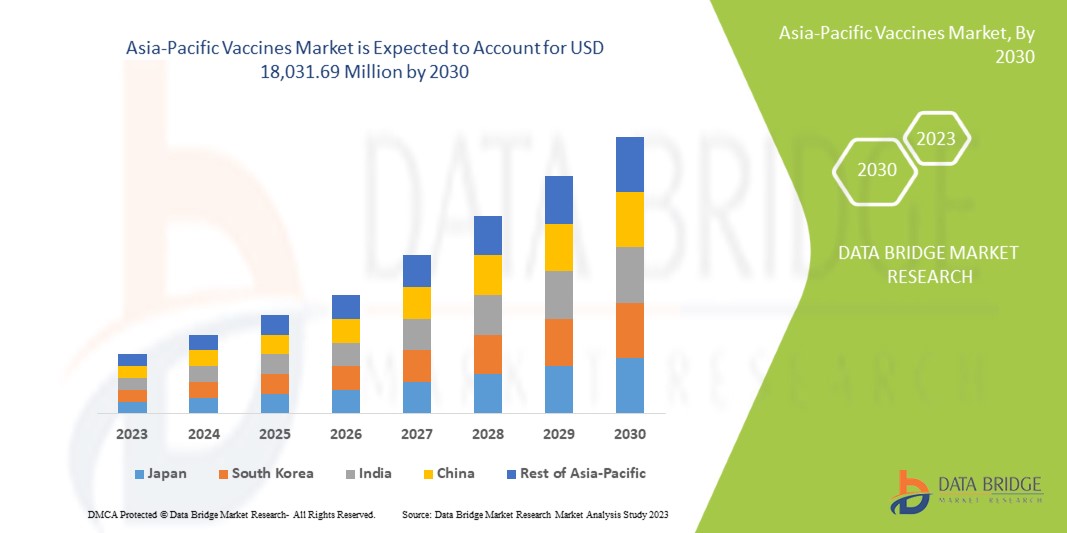
Der Impfstoffmarkt im asiatisch-pazifischen Raum wird im Prognosezeitraum 2023 bis 2030 voraussichtlich wachsen. Data Bridge Market Research analysiert, dass der Markt im Prognosezeitraum 2023 bis 2030 mit einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 9,4 % wächst und von 9.048,51 Millionen USD im Jahr 2022 auf 18.031,69 Millionen USD im Jahr 2030 ansteigen dürfte.
|
Berichtsmetrik |
Details |
|
Prognosezeitraum |
2023 bis 2030 |
|
Basisjahr |
2022 |
|
Historische Jahre |
2021 (anpassbar auf 2015–2020) |
|
Quantitative Einheiten |
Umsatz in Mio. USD |
|
Abgedeckte Segmente |
Nach Zusammensetzung (Kombinationsimpfstoffe und Monoimpfstoffe), Typ (Untereinheiten-, rekombinante, Polysaccharid- und Konjugatimpfstoffe, Lebendimpfstoffe, inaktivierte Impfstoffe und Toxoidimpfstoffe), Art (Routineimpfstoff, empfohlener Impfstoff und vorgeschriebener Impfstoff), Verabreichungsalter (Kinderimpfstoff und Erwachsenenimpfstoff), Krankheiten (Pneumokokken-Erkrankung, Masern, Mumps und Windpocken, DPT, Hepatitis, Grippe, Typhus, Meningokokken, Tollwut, Japanische Enzephalitis, Gelbfieber und andere), Verabreichungsweg (injizierbar, oral und nasal), Endverbraucher (Gemeindekrankenhäuser, Krankenhäuser, Fachzentren, Kliniken und andere), Vertriebskanal (Krankenhausapotheke, Einzelhandelsapotheke und Online-Apotheke) |
|
Abgedeckte Länder |
Japan, China, Australien, Indien, Südkorea, Singapur, Indonesien, Thailand, Malaysia, Philippinen, Vietnam und Rest des asiatisch-pazifischen Raums |
|
Abgedeckte Marktteilnehmer |
Bharat Biotech, Biological E Limited, Bio Farma, Serum Institute of India Pvt. Ltd., Takeda Pharmaceutical Company Limited, Merck Sharp & Dohme Corp. (eine Tochtergesellschaft von Merck & Co., Inc.), Abbott, AstraZeneca, Sanofi, Pfizer Inc., Janssen Global Services, LLC (eine Tochtergesellschaft von Johnson & Johnson Services, Inc.), F. Hoffmann-La Roche Ltd, Panacea Biotec Ltd und BAXTER VACCINES (eine Tochtergesellschaft von Baxter) unter anderem |
Marktdefinition des Impfstoffmarktes im asiatisch-pazifischen Raum
Impfstoffe sind Produkte, die das Immunsystem des Individuums stimulieren, um Immunität gegen eine bestimmte Krankheit zu erzeugen. Impfstoffe funktionieren nach dem Prinzip des Gedächtnisses und der Wiedererkennung. Wenn abgeschwächte oder abgetötete Mikroben in einen Körper injiziert werden, veranlassen diese Mikroben B-Zellen, Gedächtniszellen des Immunsystems, den Erreger zu erkennen. Wenn derselbe Erreger in der Zukunft den Körper angreift, wirkt er gegen diese. Es wurden Impfstoffe für Infektionskrankheiten entdeckt, darunter Pneumokokken-Erkrankungen, Masern, Mumps, Röteln, Hepatitis, Grippe, Typhus, Windpocken und Tollwut.
Es gibt zwei Arten von Impfstoffen: Kombinationsimpfstoffe (die verschiedene Stämme des Erregers enthalten) und Monoimpfstoffe (die einen einzigen Stamm des Erregers enthalten). Es wurden verschiedene Arten von Impfstoffen auf der Grundlage des aus dem Erreger extrahierten Materials entwickelt, das aus Polysaccharidhüllen, DNA, RNA und dem gesamten Organismus bestehen kann, entweder inaktiviert oder lebend.
Diese Impfstoffe haben zur Ausrottung von Krankheiten wie Polio geführt. Je nach Vorliebe und Wirksamkeit der Impfstoffe können diese auf verschiedene Arten verabreicht werden: injizieren, oral oder nasal. Die injizierbare Verabreichungsmethode wird jedoch stark bevorzugt, da sie eine systemische Reaktion hervorruft. Die Impfung kann unter anderem in Krankenhäusern, Gemeinschaftskliniken und Spezialkliniken von geschultem Personal durchgeführt werden, das über entsprechende Kenntnisse über Impfstoffverabreichungsgeräte verfügt.
Die zunehmende Verbreitung von Infektionskrankheiten, darunter bakterielle und virale Erkrankungen, beschert dem Markt lukratives Wachstum. Darüber hinaus kurbeln auch die zunehmende staatliche Unterstützung und die Einführung neuerer Impfstoffe den Impfstoffmarkt an. Ein weiterer Faktor, der das Wachstum des Impfstoffmarktes ankurbelt, ist das zunehmende Bewusstsein für Impfungen und die Nachfrage nach wirksamen COVID-19-Impfstoffen.
Dynamik des Impfstoffmarktes im asiatisch-pazifischen Raum
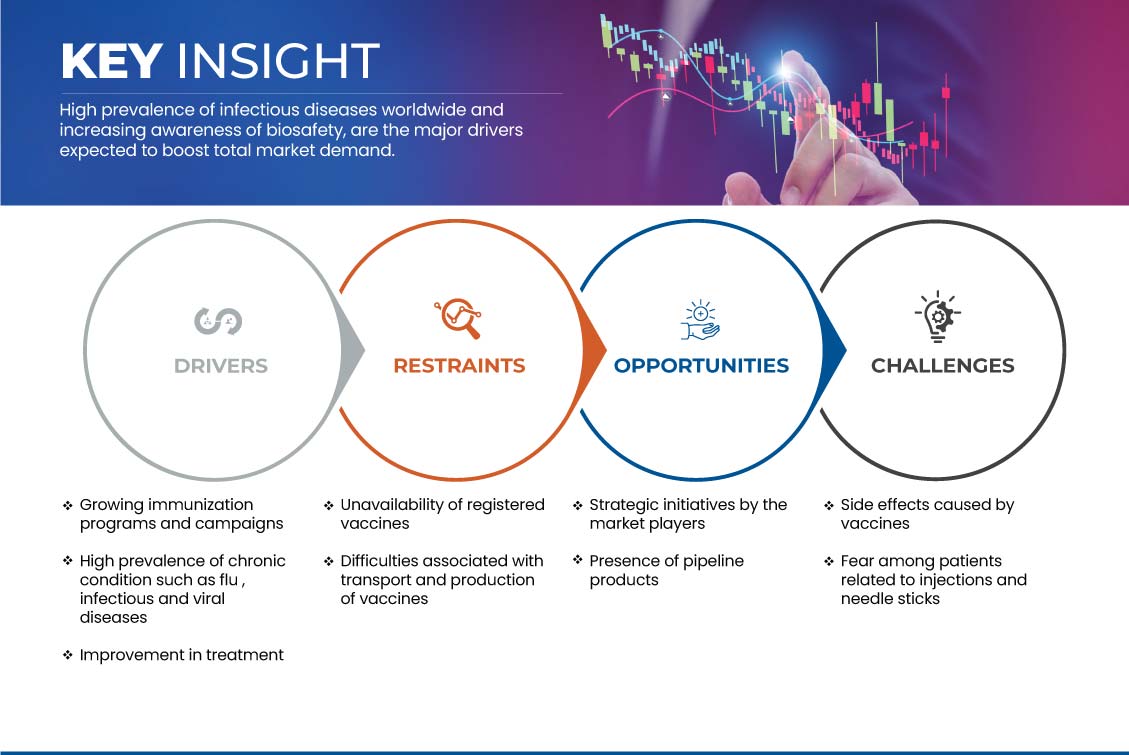
In diesem Abschnitt geht es um das Verständnis der Markttreiber, Vorteile, Chancen, Einschränkungen und Herausforderungen. All dies wird im Folgenden ausführlich erläutert:
TREIBER
WACHSTUM DER IMMUNISIERUNGSPROGRAMME UND -KAMPAGNEN
Aufgrund der steigenden Zahl chronischer Krankheiten werden weltweit immer mehr Impfprogramme und -kampagnen durchgeführt. Da Hepatitis, Diphtherie, Keuchhusten und Kinderlähmung sowie andere Infektionskrankheiten in der Umwelt weit verbreitet sind, besteht dringender Bedarf, das Bewusstsein für Impfungen zu schärfen. Dies kann durch die Einführung mehrerer Kampagnen und Programme erreicht werden. Es wurde berichtet, dass die Zahl der Impfprogramme mit der Zunahme von Infektionskrankheiten zunimmt. Auch die Impfraten nehmen weltweit zu, um schwächende Krankheiten zu bekämpfen.
Dennoch sind rund 20 Millionen Menschen noch nicht oder nicht ausreichend geimpft, was einen großen Bedarf an Impfungen nach sich zieht. Die Zahl chronischer Krankheiten nimmt weltweit zu. Daher ist der Bedarf an Impfungen groß. Dies bedeutet, dass ein wachsendes Immunisierungsprogramm und eine entsprechende Kampagne den Impfstoffmarkt voraussichtlich wachsen lassen werden.
HOHE PRÄVALENZ CHRONISCHER ERKRANKUNGEN WIE GRIPPE, INFEKTIONS- UND VIRALERKRANKUNGEN
Die Verbreitung von Infektionskrankheiten nimmt weltweit zu, und Grippe und bakterielle Infektionskrankheiten nehmen rapide zu. Diese steigende Zahl an Infektionskrankheiten hat die Notwendigkeit einer Krankheitsvorbeugung geschaffen, die durch Impfung oder Immunisierung verhindert werden kann. Da die Zahl der Krankheiten stark zunimmt, besteht dringender Bedarf an Massenimpfungen. Massenimpfungen erfordern viele Krankheiten und dürften daher dem Impfstoffmarkt lukratives Wachstum bescheren.
Da die Zahl der Krankheitsfälle zunimmt, wird auch der Schwerpunkt auf Massenimpfungen gelegt, um eine große Bevölkerungszahl zu verhindern. Viele Menschen, die in jüngeren Jahren nicht geimpft wurden, sind weltweit immunisiert. Um diesen Bedarf zu decken, steigt die Nachfrage nach neuartigen Impfstoffen und wird daher voraussichtlich als Treiber für den Impfstoffmarkt im asiatisch-pazifischen Raum wirken.
ZURÜCKHALTUNG
NICHTVERFÜGBARKEIT REGISTRIERTER IMPFSTOFFE
Die strengen behördlichen Genehmigungen und die zeitaufwändigen Entwicklungsverfahren für die Impfstoffe sind einige der Faktoren, die für die Nichtverfügbarkeit zugelassener Impfstoffe verantwortlich sind. Die Zulassungsbehörden haben Schwierigkeiten bei der Bewertung, Zulassung, Kontrolle und Überwachung der Impfstoffe. Die weltweite Versorgung mit Impfstoffen verzögert sich aufgrund dieser Vorschriften.
Daher können die Regulierungsprozesse und die Dokumentenerstellung der Herstellerunternehmen in verschiedenen Ländern eine einschränkende Wirkung haben und das Wachstum des Impfstoffmarktes behindern.
GELEGENHEIT
STRATEGISCHE INITIATIVEN DER MARKTPLATZ
Marktteilnehmer ergreifen auf dem Impfstoffmarkt verschiedene strategische Initiativen, die Expansion, Zusammenarbeit und Akquisition beinhalten. Diese Initiativen ermöglichen es ihnen, das Produktportfolio des Unternehmens zu erweitern, was zu einer Marktexpansion führt und die Produktnachfrage bei den Kunden steigert, was den Marktteilnehmern letztendlich ermöglicht, maximale Einnahmen zu erzielen.
Da die Nachfrage nach wirksamen und neuartigen Impfstoffen weltweit steigt, zielen diese strategischen Initiativen der führenden Marktteilnehmer darauf ab, die Geschäftsabläufe zu verbessern und die Rentabilität des Marktes zu steigern.
Verschiedene strategische Initiativen der Marktteilnehmer ermöglichten es ihnen, ihre Präsenz im Impfstoffbereich auszubauen und mehr Marktwachstum zu erzielen. Daher ergreifen die Marktteilnehmer im Impfstoffbereich mehrere strategische Initiativen, von denen erwartet wird, dass sie dem Wachstum des Impfstoffmarktes eine Chance bieten.
HERAUSFORDERUNG
Nebenwirkungen durch Impfungen
Der Impfstoff ist ein medizinisches Produkt, das bei der Vorbeugung verschiedener Krankheiten hilft. Manchmal treten jedoch durch die Verwendung der Impfstoffe Nebenwirkungen auf. Einige davon sind leichte Nebenwirkungen wie Rötung, Schmerzen oder Schwellung an der Injektionsstelle. Die Nebenwirkungen der Impfstoffe sind jedoch selten, aber lebensbedrohlich.
Die lebensbedrohlichen Nebenwirkungen können in der Bevölkerung Angst auslösen. Darüber hinaus beeinträchtigen sie die Glaubwürdigkeit der Impfstoffhersteller und wirken sich auf den Produktabsatz aus. Dies lässt darauf schließen, dass durch Impfstoffe verursachte Nebenwirkungen das Wachstum des Impfstoffmarktes behindern können.
Jüngste Entwicklungen
- Im Oktober 2022 brachte Indonesien seinen ersten inländischen Impfstoff gegen COVID-19 auf den Markt. Der Impfstoff IndoVac wurde gemeinsam vom staatlichen indonesischen Pharmaunternehmen Bio Farma und dem Baylor College of Medicine, einem unabhängigen Zentrum für Gesundheitswissenschaften in Houston, Texas, entwickelt.
- Im November 2020 unterzeichnete Merck Sharp & Dohme Corp., eine Tochtergesellschaft von Merck & Co., Inc., eine Vereinbarung zur Übernahme von OncoImmune, einem biopharmazeutischen Unternehmen in der klinischen Phase. Das Unternehmen OncoImmune konzentriert sich stark auf die Entwicklung von Behandlungsmöglichkeiten für COVID-19. Durch diese Vereinbarung erwartet das Unternehmen die Entwicklung neuer Impfstoffkandidaten
- Im September 2020 unterzeichneten Sanofi und GSK eine Vereinbarung mit der kanadischen Regierung über die Lieferung von 72 Millionen Dosen COVID-19-Impfstoffen. Es besteht eine hohe Nachfrage nach COVID-19-Impfstoffen, und die Nachfrage steigt mit der zunehmenden Pandemie. Diese Vereinbarung ermöglichte es dem Unternehmen, zukünftiges Potenzial sicherzustellen
Marktumfang für Impfstoffe im asiatisch-pazifischen Raum
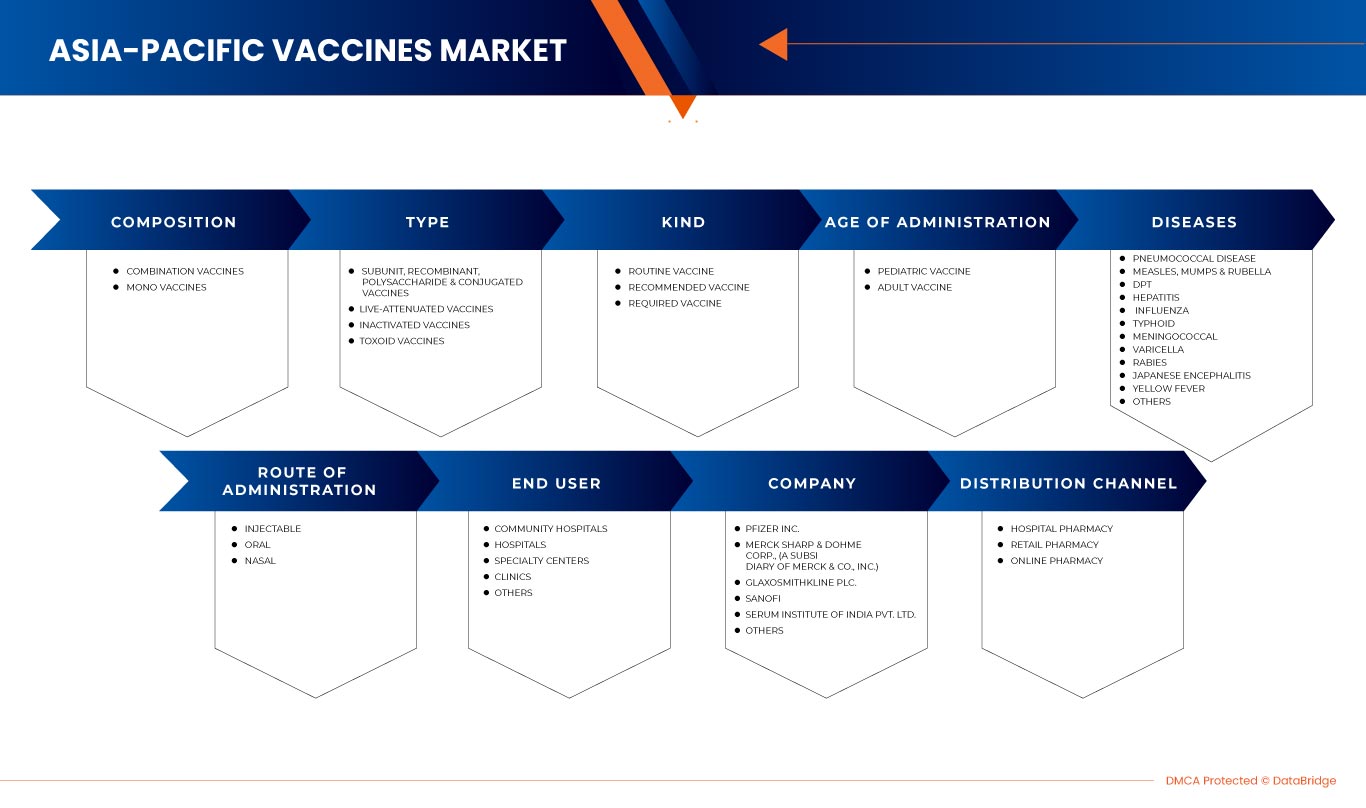
Der Impfstoffmarkt im asiatisch-pazifischen Raum ist segmentiert nach Zusammensetzung, Art, Art, Verabreichungsalter, Krankheiten, Verabreichungsweg, Endverbraucher und Vertriebskanal. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern einen wertvollen Marktüberblick und Markteinblicke, um strategische Entscheidungen zur Identifizierung der wichtigsten Marktanwendungen zu treffen.
ZUSAMMENSETZUNG
- Kombinationsimpfstoffe
- Monoimpfstoffe
Auf Grundlage seiner Zusammensetzung ist der Impfstoffmarkt im asiatisch-pazifischen Raum in Kombinationsimpfstoffe und Monoimpfstoffe segmentiert.
TYP
- Untereinheiten-, rekombinante, Polysaccharid- und Konjugatimpfstoffe
- Lebendimpfstoffe
- Inaktivierte Impfstoffe
- Toxoid-Impfstoffe
On the basis of type, the Asia-Pacific vaccines market is segmented into subunit, recombinant, polysaccharide, and conjugate vaccines, live-attenuated vaccines, inactivated vaccines, and toxoid vaccines.
KIND
- Routine vaccine
- Recommended vaccine
- Required vaccine
On the basis of kind, the Asia-Pacific vaccines market is segmented into routine vaccine, recommended vaccine, and required vaccine.
AGE OF ADMINISTRATION
- Pediatric vaccine
- Adult vaccine
On the basis of age of administration, the Asia-Pacific vaccines market is segmented into pediatric vaccine and adult vaccine
DISEASES
- Pneumococcal disease
- Measles, mumps & varicella
- DPT
- Hepatitis
- Influenza
- Typhoid
- Meningococcal
- Varicella
- Rabies
- Japanese encephalitis
- Yellow fever
- Others
On the basis of diseases, the Asia-Pacific vaccines market is segmented into pneumococcal disease, measles, mumps, and varicella, DPT, hepatitis, influenza, typhoid, meningococcal, rabies, Japanese encephalitis, yellow fever, and others.
ROUTE OF ADMINISTRATION
- Injectable
- Nasal
- Oral
On the basis of route of administration, the Asia-Pacific vaccines market is segmented into injectable, oral, and nasal.
END USER
- Community hospitals
- Hospitals
- Specialty centres
- Clinics
- Others
On the basis of end user, the Asia-Pacific vaccines market is segmented into community hospitals, hospitals, specialty centres, clinics, and others.
DISTRIBUTION CHANNEL
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
On the basis of distribution channel, the Asia-Pacific vaccines market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy.
Asia-Pacific Vaccines Market Regional Analysis/Insights
Asia-Pacific vaccines market is analyzed, and market size insights and trends are provided by country, composition, type, kind, age of administration, diseases, route of administration, end user, and distribution channel, as referenced above.
The countries covered in this market report are Japan, China, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Vietnam, and the rest of Asia-Pacific.
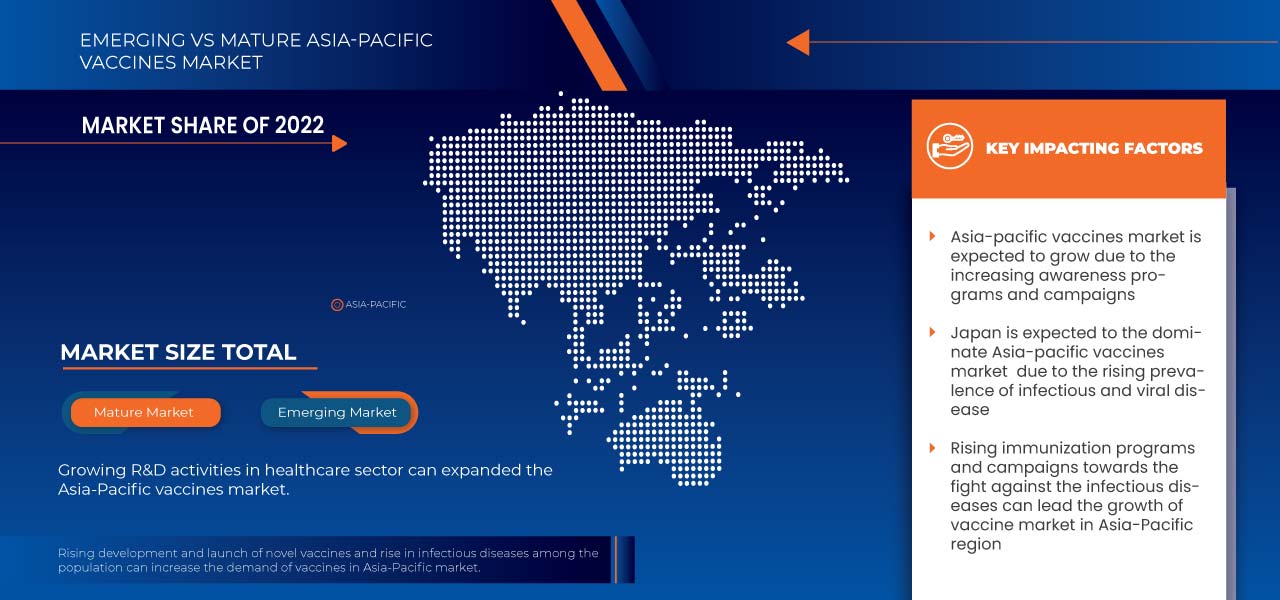
Japan is expected to dominate the Asia-Pacific vaccine market regarding market share and revenue and will continue to flourish its dominance during the forecast period. This is due to a rising preference for preventive health check-ups.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of Global brands and their challenges faced due to competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Asia-Pacific Vaccines Market Share Analysis
Die Wettbewerbslandschaft des Impfstoffmarktes liefert Einzelheiten zu einem Wettbewerber. Zu den Einzelheiten gehören Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben genannten Datenpunkte beziehen sich nur auf den Fokus der Unternehmen auf den Impfstoffmarkt.
Zu den wichtigsten Akteuren auf dem Impfstoffmarkt im asiatisch-pazifischen Raum zählen unter anderem Bharat Biotech, Biological E Limited, Bio Farma, Serum Institute of India Pvt. Ltd., Takeda Pharmaceutical Company Limited, Merck Sharp & Dohme Corp. (eine Tochtergesellschaft von Merck & Co., Inc.), Abbott, AstraZeneca, Sanofi, Pfizer Inc., Janssen Global Services, LLC (eine Tochtergesellschaft von Johnson & Johnson Services, Inc.), F. Hoffmann-La Roche Ltd, Panacea Biotec Ltd und BAXTER VACCINES (eine Tochtergesellschaft von Baxter).
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC VACCINES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 COMPOSITION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL MODEL
4.2 PORTER'S FIVE FORCES
4.3 EPIDEMIOLOGY
4.4 INDUSTRIAL INSIGHTS:
4.5 PIPELINE ANALYSIS
4.6 ASIA-PACIFIC VACCINES MARKET: SUPPLY CHAIN MANAGEMENT OF VACCINES
4.6.1 COLD CHAIN STORAGE:
4.6.2 PROCESS OF LOGISTICS
5 REGULATORY FRAMEWORK
5.1 JAPAN
5.2 CHINA
5.3 SOUTH KOREA
5.4 INDIA
5.5 AUSTRALIA
5.6 SINGAPORE
5.7 THAILAND
5.8 MALAYSIA
5.9 INDONESIA
5.1 VIETNAM
5.11 PHILIPPINES
5.12 REST OF ASIA-PACIFIC
5.12.1 TAIWAN
5.12.2 CAMBODIA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS
6.1.2 HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU, INFECTIOUS AND VIRAL DISEASES
6.1.3 IMPROVEMENT IN TREATMENT
6.1.4 LAUNCH OF NEWER VACCINES
6.1.5 INCREASING GOVERNMENT SUPPORT
6.2 RESTRAINTS
6.2.1 UNAVAILABILITY OF REGISTERED VACCINES
6.2.2 DIFFICULTIES ASSOCIATED WITH THE TRANSPORT AND PRODUCTION OF VACCINES
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY THE MARKET PLAYERS
6.3.2 PRESENCE OF PIPELINE PRODUCTS
6.3.3 RISE IN EXPENDITURE IN THE HEALTHCARE SECTOR
6.3.4 INCREASING AWARENESS FOR VACCINATION
6.4 CHALLENGES
6.4.1 SIDE EFFECTS CAUSED BY VACCINES
6.4.2 FEAR AMONG PATIENTS RELATED TO INJECTIONS AND NEEDLE STICKS
6.4.3 PRODUCT RECALL
7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION
7.1 OVERVIEW
7.2 COMBINATION VACCINES
7.3 MONO VACCINES
8 ASIA-PACIFIC VACCINES MARKET, BY TYPE
8.1 OVERVIEW
8.2 SUBUNIT, RECOMBINANT, POLYSACCHARIDE, AND CONJUGATE VACCINES
8.2.1 PNEUMOCOCCAL DISEASE
8.2.2 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
8.2.3 HPV (HUMAN PAPILLOMA VIRUS)
8.2.4 HEPATITIS B
8.2.5 MENINGOCOCCAL
8.2.6 SHINGLES
8.2.7 WHOOPING COUGH
8.2.8 OTHERS
8.3 LIVE-ATTENUTAED VACCINES
8.3.1 ROTAVIRUS
8.3.2 MEASLES
8.3.3 MUMPS
8.3.4 RUBELLA
8.3.5 SMALLPOX
8.3.6 YELLOW FEVER
8.3.7 OTHERS
8.4 INACTIVATED VACCINES
8.4.1 FLU (SHOT ONLY)
8.4.2 POLIO (SHOT ONLY)
8.4.3 HEPATITIS A
8.4.4 RABIES
8.4.5 OTHERS
8.5 TOXOID VACCINES
8.5.1 DIPHTHERIA, TETANUS & PERTUSSIS (DTP)
8.5.2 OTHERS
9 ASIA-PACIFIC VACCINES MARKET, BY KIND
9.1 OVERVIEW
9.2 ROUTINE VACCINES
9.2.1 PNEUMOCOCCAL DISEASES
9.3 DIPTHERIA, TETANUS & PERTUSIS(DPT)
9.3.1 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
9.3.2 MEASLES
9.3.3 MUMPS
9.3.4 HEPATITIS B
9.3.5 RUBELLA
9.3.6 POLIO
9.3.7 OTHERS
9.4 RECOMMENDED VACCINE
9.4.1 TYPHOID FEVER VACCINE
9.5 HEPATITIS A
9.5.1 RABIES
9.5.2 JAPANESE ENCEPHALITIS
9.5.3 TICK-BORNE ENCEPHALITIS
9.5.4 CHOLERA
9.5.5 OTHERS
9.6 REQUIRED VACCINE
9.6.1 MENINGOCOCCAL
9.7 YELLOW FEVER
9.7.1 OTHERS
10 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION
10.1 OVERVIEW
10.2 PEDIATRIC VACCINE
10.2.1 PNEUMOCOCCAL DISEASES
10.3 MEASLES, MUMPS & RUBELLA
10.3.1 DIPTHERIA, TETANUS & PERTUSIS (DPT)
10.3.2 ROTAVIRUS
10.3.3 MENINGOCOCCAL
10.3.4 VARICELLA
10.3.5 POLIO
10.3.6 TUBERCULOSIS
10.3.7 MALARIA
10.3.8 OTHERS
10.4 ADULT VACCINE
10.4.1 INFLUENZA
10.5 HPV (HUMAN PAPILLOMA VIRUS)
10.5.1 TYPHOID
10.5.2 HEPATITIS B
10.5.3 JAPANESE ENCEPHALITIS
10.5.4 YELLOW FEVER
10.5.5 CANCER
10.5.6 OTHERS
11 ASIA-PACIFIC VACCINES MARKET, BY DISEASES
11.1 OVERVIEW
11.2 PNEUMOCCOCAL DISEASE
11.3 MEASLES, MUMPS & RUBELLA
11.4 DPT
11.5 HEPATITIS
11.6 INFLUENZA
11.7 TYPHOID
11.8 MENINGOCOCCAL
11.9 VARICELLA
11.1 RABIES
11.11 JAPANESE ENCEPHALITIS
11.12 YELLOW FEVER
11.13 OTHERS
12 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 INJECTABLE
12.2.1 INTRAMUSCULAR
12.2.2 SUBCUTANEOUS
12.2.3 INTRADERMAL
12.3 ORAL
12.4 NASAL
13 ASIA-PACIFIC VACCINES MARKET, BY END USER
13.1 OVERVIEW
13.2 COMMUNITY HOSPITALS
13.3 HOSPITALS
13.4 SPECIALTY CENTERS
13.5 CLINICS
13.6 OTHERS
14 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 ASIA-PACIFIC VACCINE MARKET
15.1 ASIA-PACIFIC
15.1.1 JAPAN
15.1.2 CHINA
15.1.3 AUSTRALIA
15.1.4 INDIA
15.1.5 SOUTH KOREA
15.1.6 SINGAPORE
15.1.7 MALAYSIA
15.1.8 THAILAND
15.1.9 INDONESIA
15.1.10 PHILIPPINES
15.1.11 VIETNAM
15.1.12 REST OF ASIA PACIFIC
16 ASIA-PACIFIC VACCINES MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
16.2 DISEASE SHARE ANALYSIS: PFIZER, INC.
16.3 COUNTRY SHARE ANALYSIS: PFIZER, INC.
16.4 DISEASE SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.5 COUNTRY SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.6 DISEASE SHARE ANALYSIS: GLAXOSMITHKLINE PLC
16.7 COUNTRY SHARE ANALYSIS: GLAXOSMITHKLINE PLC.
16.8 DISEASE SHARE ANALYSIS: SANOFI
16.9 COUNTRY SHARE ANALYSIS: SANOFI
16.1 DISEASE SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
16.11 COUNTRY SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 PFIZER INC.
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY WEBSITE AND PRESS RELEASES
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GLAXOSMITHKLINE PLC.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 PRODUCT PORTFOLIO
18.3.4 RECENT DEVELOPMENTS
18.4 SANOFI
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 PRODUCT PORTFOLIO
18.4.4 RECENT DEVELOPMENTS
18.5 SERUM INSTITUTE OF INDIA PVT. LTD.
18.5.1 COMPANY SNAPSHOT
18.5.2 PRODUCT PORTFOLIO
18.5.3 RECENT DEVELOPMENTS
18.6 ABBOTT
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 ASTRAZENECA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENTS
18.8 ALK
18.8.1 COMPANY SNAPSHOT
18.8.2 REVENUE ANALYSIS
18.8.3 PRODUCT PORTFOLIO
18.8.4 RECENT DEVELOPMENTS
18.9 BAXTER VACCINES (A SUBSIDIARY OF BAXTER)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BHARAT BIOTECH
18.10.1 COMPANY SNAPSHOT
18.10.2 PRODUCT PORTFOLIO
18.10.3 RECENT DEVELOPMENTS
18.11 BIO FARMA
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENTS
18.12 BIOLOGICAL E LIMITED
18.12.1 COMPANY SNAPSHOT
18.12.2 PRODUCT PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 DAIICHI SANKYO COMPANY, LIMITED
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENTS
18.14 F. HOFFMANN-LA ROCHE LTD
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 JANSSEN GLOBAL SERVICES, LLC (A SUBSIDIARY OF JOHNSON & JOHNSON SERVICES, INC.)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENTS
18.16 LANZHOU BIOLOGICAL PRODUCTS RESEARCH INSTITUTE CO., LTD.,
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENTS
18.17 PANACEA BIOTEC LTD
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENTS
18.18 SEQIRUS (A SUBSIDIARY OF CSL LIMITED)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENTS
18.19 TAKEDA PHARMACEUTICAL COMPANY LIMITED
18.19.1 COMPANY SNAPSHOT
18.19.2 REVENUE ANALYSIS
18.19.3 COMPANY WEBSITE AND PRESS RELEASES
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
Tabellenverzeichnis
TABLE 1 ASIA-PACIFIC VACCINES MARKET, PIPELINE ANALYSIS
TABLE 2 RECOMMENDED TEMPERATURE AND STORAGE LENGTH AT VARIOUS LEVELS OF THE COLD CHAIN.
TABLE 3 LOGISTICS PROCESS ACROSS DIFFERENT REGIONS.
TABLE 4 LAWS AND REGULATIONS IN TAIWAN
TABLE 5 VACCINES UNDER CLINICAL TRIAL
TABLE 6 THE SIDE EFFECTS RELATED TO THE VACCINES
TABLE 7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 8 ASIA-PACIFIC VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 9 ASIA-PACIFIC SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 ASIA-PACIFIC LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 ASIA-PACIFIC INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 ASIA-PACIFIC TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 ASIA-PACIFIC VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 14 ASIA-PACIFIC ROUTINE VACCINES IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 15 ASIA-PACIFIC RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 16 ASIA-PACIFIC REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 17 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 18 ASIA-PACIFIC PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 19 ASIA-PACIFIC ADULT VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 20 ASIA-PACIFIC VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 21 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 22 ASIA-PACIFIC INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 23 ASIA-PACIFIC VACCINES MARKET, BY END USER, 2022-2030 (USD MILLION)
TABLE 24 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 25 ASIA-PACIFIC KNEE CARTILAGE REPAIR MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 26 JAPAN VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 27 JAPAN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 JAPAN LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 JAPAN INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 JAPAN TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 33 JAPAN ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 34 JAPAN RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 35 JAPAN REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 36 JAPAN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 37 JAPAN PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 JAPAN ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 JAPAN VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 40 JAPAN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 JAPAN INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 JAPAN VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 43 JAPAN VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 44 CHINA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 45 CHINA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 46 CHINA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 47 CHINA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 48 CHINA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 49 CHINA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 50 CHINA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 51 CHINA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 52 CHINA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 53 CHINA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 54 CHINA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 55 CHINA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 56 CHINA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 57 CHINA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 58 CHINA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 59 CHINA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 60 CHINA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 61 CHINA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 AUSTRALIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 63 AUSTRALIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 64 AUSTRALIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 65 AUSTRALIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 66 AUSTRALIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 67 AUSTRALIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 AUSTRALIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 69 AUSTRALIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 70 AUSTRALIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 71 AUSTRALIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 72 AUSTRALIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 73 AUSTRALIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 74 AUSTRALIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 AUSTRALIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 76 AUSTRALIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 77 AUSTRALIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 78 AUSTRALIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 79 INDIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 80 INDIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 INDIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 INDIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 INDIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 INDIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 INDIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 86 INDIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 87 INDIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 88 INDIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 89 INDIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 90 INDIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 91 INDIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 92 INDIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 93 INDIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 94 INDIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 95 INDIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 96 INDIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 97 SOUTH KOREA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 98 SOUTH KOREA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 99 SOUTH KOREA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 100 SOUTH KOREA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 101 SOUTH KOREA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 102 SOUTH KOREA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 103 SOUTH KOREA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 104 SOUTH KOREA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 105 SOUTH KOREA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 106 SOUTH KOREA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 107 SOUTH KOREA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 108 SOUTH KOREA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 109 SOUTH KOREA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 110 SOUTH KOREA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SOUTH KOREA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SOUTH KOREA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SOUTH KOREA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 SINGAPORE VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 115 SINGAPORE SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 SINGAPORE LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 SINGAPORE INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 SINGAPORE TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 SINGAPORE VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 120 SINGAPORE ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 121 SINGAPORE RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 122 SINGAPORE REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 123 SINGAPORE VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 124 SINGAPORE PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 125 SINGAPORE ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 126 SINGAPORE VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 127 SINGAPORE VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 128 SINGAPORE INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 129 SINGAPORE VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 SINGAPORE VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 131 MALAYSIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 132 MALAYSIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 MALAYSIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 MALAYSIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 135 MALAYSIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 136 MALAYSIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 137 MALAYSIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 138 MALAYSIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 139 MALAYSIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 140 MALAYSIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 141 MALAYSIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 142 MALAYSIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 143 MALAYSIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 144 MALAYSIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 145 MALAYSIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 146 MALAYSIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 MALAYSIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 148 MALAYSIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 149 THAILAND VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 150 THAILAND VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 151 THAILAND SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 THAILAND LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 THAILAND INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 THAILAND TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 THAILAND VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 156 THAILAND RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 157 THAILAND REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 158 THAILAND VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 THAILAND PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 THAILAND ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 161 THAILAND VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 162 THAILAND VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 163 THAILAND INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 164 THAILAND VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 THAILAND VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 INDONESIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 167 INDONESIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 INDONESIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 INDONESIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 INDONESIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 171 INDONESIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 172 INDONESIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 173 INDONESIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 174 INDONESIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 175 INDONESIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 176 INDONESIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 177 INDONESIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 178 INDONESIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 179 INDONESIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 180 INDONESIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 181 INDONESIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 182 INDONESIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 183 INDONESIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 184 PHILIPPINES VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 185 PHILIPPINES VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 186 PHILIPPINES SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 187 PHILIPPINES LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 PHILIPPINES INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 PHILIPPINES TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 PHILIPPINES VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 191 PHILIPPINES ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 192 PHILIPPINES RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 193 PHILIPPINES REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 194 PHILIPPINES VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 PHILIPPINES PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 PHILIPPINES ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 197 PHILIPPINES VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 198 PHILIPPINES VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 199 PHILIPPINES INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 200 PHILIPPINES VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 201 PHILIPPINES VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 202 VIETNAM VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 203 VIETNAM VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 VIETNAM SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 VIETNAM LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 VIETNAM INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 207 VIETNAM TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 208 VIETNAM VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 209 VIETNAM ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 210 VIETNAM RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 211 VIETNAM REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 212 VIETNAM VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 213 VIETNAM PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 214 VIETNAM ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 215 VIETNAM VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 216 VIETNAM VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 217 VIETNAM INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 218 VIETNAM VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 219 VIETNAM VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 220 REST OF ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
Abbildungsverzeichnis
FIGURE 1 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC VACCINES MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC VACCINES MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC VACCINES MARKET: ASIA-PACIFIC VS COUNTRY MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC VACCINES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC VACCINES MARKET: MULTIVARIATE MODELLING
FIGURE 7 ASIA-PACIFIC VACCINES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 ASIA-PACIFIC VACCINES MARKET: DBMR MARKET POSITION GRID
FIGURE 9 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 10 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS AND THE HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU AND BACTERIAL INFECTIOUS DISEASES ARE DRIVING THE ASIA-PACIFIC VACCINES MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 11 THE COMBINATION VACCINES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC VACCINES MARKET IN 2023 & 2030
FIGURE 12 FDA REGULATORY REVIEW PROCESS OF VACCINES
FIGURE 13 PROCESS OF SPECIAL APPROVAL ON VACCINES DURING THE 2019 H1N1PDM PANDEMIC
FIGURE 14 REGULATION OVERVIEW FOR THERAPEUTICS IN SINGAPORE
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF THE ASIA-PACIFIC VACCINES MARKET
FIGURE 16 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2022
FIGURE 17 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2023-2030 (USD MILLION)
FIGURE 18 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, CAGR (2023-2030)
FIGURE 19 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, LIFELINE CURVE
FIGURE 20 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2022
FIGURE 21 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 22 ASIA-PACIFIC VACCINES MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 23 ASIA-PACIFIC VACCINES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2022
FIGURE 25 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2023-2030 (USD MILLION)
FIGURE 26 ASIA-PACIFIC VACCINES MARKET: BY KIND, CAGR (2023-2030)
FIGURE 27 ASIA-PACIFIC VACCINES MARKET: BY KIND, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2022
FIGURE 29 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 30 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 31 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2022
FIGURE 33 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2023-2030 (USD MILLION)
FIGURE 34 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, CAGR (2023-2030)
FIGURE 35 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 37 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 38 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 39 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 40 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2022
FIGURE 41 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 ASIA-PACIFIC VACCINES MARKET: BY END USER, CAGR (2023-2030)
FIGURE 43 ASIA-PACIFIC VACCINES MARKET: BY END USER, LIFELINE CURVE
FIGURE 44 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 ASIA-PACIFIC VACCINE MARKET: SNAPSHOT (2022)
FIGURE 49 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022)
FIGURE 50 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 ASIA-PACIFIC VACCINE MARKET: BY COMPOSITION (2023-2030)
FIGURE 53 ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 54 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 55 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 56 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 57 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 58 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 59 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 60 SANOFI, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 61 SANOFI ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 62 SERUM INSTITUTE OF INDIA PVT. LTD., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 63 SERUM INSTITUTE OF INDIA PVT. LTD. ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.