Markt für interventionelle neurologische Geräte im asiatisch-pazifischen Raum, nach Produkttyp (Geräte zum Coiling und Embolisieren von Aneurysmen, Systeme zur zerebralen Ballonangioplastie und Stenteinlage, Stützgeräte, Geräte zur Neurothrombektomie), Krankheitspathologie (ischämische Schlaganfälle, zerebrale Aneurysmen, arteriovenöse Malformationen und Fisteln, sonstige), Verfahren (Embolisierung, Angioplastie, Neurothrombektomie, sonstige), Endbenutzer (Krankenhäuser, neurologische Kliniken, ambulante Pflegezentren, sonstige), Land (Japan, China, Indien, Südkorea, Australien, Singapur, Malaysia, Thailand, Indonesien, Philippinen, übriger asiatisch-pazifischer Raum) – Branchentrends und Prognose bis 2029.
Marktanalyse und Einblicke: Markt für interventionelle neurologische Geräte im asiatisch-pazifischen Raum
Der Markt für interventionelle neurologische Geräte wird im Prognosezeitraum von 2022 bis 2029 voraussichtlich ein Marktwachstum von 15,1 % verzeichnen. Der Bericht von Data Bridge Market Research zum Markt für interventionelle neurologische Geräte bietet Analysen und Erkenntnisse zu den verschiedenen Faktoren, die voraussichtlich im gesamten Prognosezeitraum vorherrschen werden, und gibt Aufschluss über ihre Auswirkungen auf das Marktwachstum. Der Aufstieg im Luftfahrtsektor beschleunigt das Wachstum des Marktes für interventionelle neurologische Geräte.
Geräte der interventionellen Neurologie werden zur nichtinvasiven und bildgesteuerten Behandlung neurovaskulärer Erkrankungen wie arteriovenöser Missbildungen , Aneurysmen, Schlaganfällen und kavernöser Missbildungen eingesetzt.
Die Prävalenz neurologischer Erkrankungen wie Alzheimer, zerebrovaskulärer Erkrankungen wie Schlaganfall, Migräne und anderer Kopfschmerzerkrankungen nimmt zu, was zu einer höheren Nachfrage nach dem Produkt führt und das Wachstum interventioneller neurologischer Geräte fördert. Große Zielpatientenpopulationen, hohe Stresslevel bei jungen Menschen und Kopfverletzungen sowie eine steigende Marktnachfrage nach wirksamen neurovaskulären Geräten sind die Hauptfaktoren, die das Wachstum des Zielmarktes vorantreiben. Darüber hinaus sind das schnelle Wachstum der geriatrischen Bevölkerung, die laufende Produktentwicklung und -vermarktung, der Ausbau der Gesundheitsinfrastruktur in den Schwellenmärkten und günstige medizinische Erstattungen die Haupttreiber, die das Wachstum des Marktes interventioneller neurologischer Geräte fördern werden.
Darüber hinaus werden der Anstieg der Nachfrage nach minimalinvasiven neurochirurgischen Verfahren und die zunehmenden Forschungsaktivitäten im Bereich neurovaskulärer Therapien dem Markt für interventionelle neurologische Geräte günstige Möglichkeiten für ein Wachstum bieten.
Der Mangel an qualifizierten Neurologen und strenge staatliche Vorschriften sind jedoch Faktoren, die das Marktwachstum behindern werden. Auch die hohen Verfahrenskosten und das Fehlen günstiger Erstattungsrichtlinien werden den Markt für interventionelle neurologische Geräte vor Herausforderungen stellen.
Dieser Marktbericht für interventionelle neurologische Geräte enthält Einzelheiten zu neuen Entwicklungen, Handelsvorschriften, Import-/Exportanalysen, Produktionsanalysen, Optimierung der Wertschöpfungskette, Marktanteilen, Auswirkungen inländischer und lokaler Marktteilnehmer, analysiert Chancen in Bezug auf neue Einnahmequellen, Änderungen der Marktvorschriften, strategische Marktwachstumsanalysen, Marktgröße, Kategoriemarktwachstum, Anwendungsnischen und -dominanz, Produktzulassungen, Produkteinführungen, geografische Expansionen und technologische Innovationen auf dem Markt. Um weitere Informationen zum Markt für interventionelle neurologische Geräte zu erhalten, wenden Sie sich an Data Bridge Market Research, um einen Analyst Brief zu erhalten. Unser Team hilft Ihnen dabei, eine fundierte Marktentscheidung zu treffen, um Marktwachstum zu erzielen.
Marktumfang und Marktgröße für interventionelle neurologische Geräte im asiatisch-pazifischen Raum
Der Markt für interventionelle neurologische Geräte ist nach Produkttyp, Krankheitsverlauf, Verfahren und Endnutzer segmentiert. Das Wachstum dieser Segmente hilft Ihnen bei der Analyse schwacher Wachstumssegmente in den Branchen und bietet den Benutzern wertvolle Marktübersichten und Markteinblicke, die ihnen bei der strategischen Entscheidungsfindung zur Identifizierung der wichtigsten Marktanwendungen helfen.
- Basierend auf dem Produkttyp ist der Markt für interventionelle neurologische Geräte segmentiert in Geräte zum Coiling und Embolisieren von Aneurysmen, zerebrale Ballonangioplastie und Stentsysteme, Stützgeräte und Geräte zur Neurothrombektomie. Das Segment der Geräte zum Coiling und Embolisieren von Aneurysmen ist weiter unterteilt in Embolie-Coils, Flussumleitungsgeräte und flüssige Emboliemittel. Embolie-Coils sind unterteilt in bloße abnehmbare Coils und beschichtete abnehmbare Coils. Das Segment der zerebralen Ballonangioplastie und Stentsysteme ist weiter unterteilt in Halsschlagader-Stents und Embolieschutzsysteme. Embolieschutzsysteme sind unterteilt in distale Filtergeräte und Ballonokklusionsgeräte. Das Segment der Stützgeräte ist weiter unterteilt in Mikroführungsdrähte und Mikrokatheter. Das Segment der Neurothrombektomiegeräte ist weiter unterteilt in Geräte zur Blutgerinnsel-Entfernung, Saug- und Aspirationsgeräte und Schlingen.
- Auf der Grundlage der Krankheitspathologie ist der Markt für interventionelle neurologische Geräte in ischämische Schlaganfälle, zerebrale Aneurysmen, arteriovenöse Missbildungen und Fisteln und andere unterteilt.
- Auf der Grundlage des Verfahrens ist der Markt für interventionelle neurologische Geräte in Embolisation, Angioplastie, Neurothrombektomie und andere unterteilt.
- Basierend auf dem Endbenutzer ist der Markt für interventionelle neurologische Geräte in Krankenhäuser, neurologische Kliniken, ambulante Pflegezentren und andere unterteilt.
Geräte für interventionelle Neurologie Markt – Länderebene Analyse
Der Markt für interventionelle neurologische Geräte wird analysiert und es werden Einblicke in die Marktgröße und Trends nach Land, Produkttyp, Krankheitspathologie, Verfahren und Endbenutzer wie oben angegeben bereitgestellt.
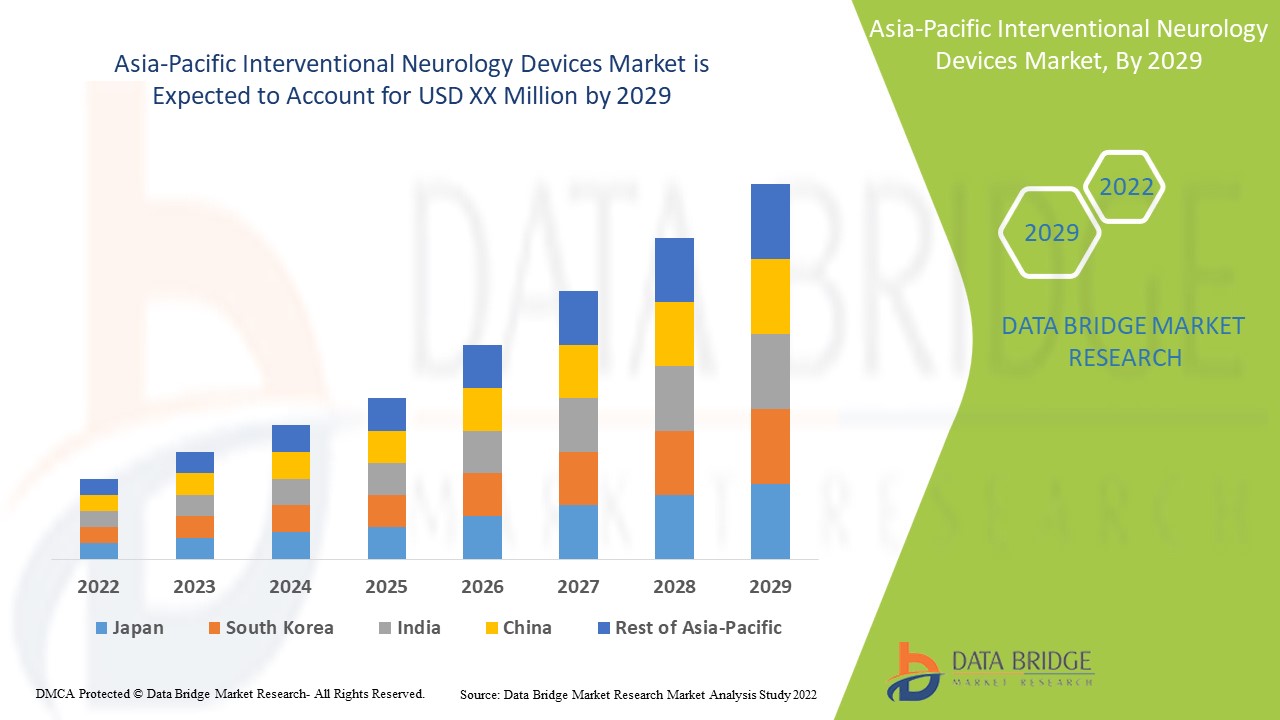
Die im Marktbericht für interventionelle neurologische Geräte abgedeckten Länder sind Japan, China, Indien, Südkorea, Australien, Singapur, Malaysia, Thailand, Indonesien, die Philippinen und der restliche asiatisch-pazifische Raum.
Japan dominiert den Markt für interventionelle neurologische Geräte in Bezug auf Marktanteil und Marktumsatz und wird seine Dominanz im Prognosezeitraum weiter ausbauen. Dies ist auf die Präsenz wichtiger Hauptakteure und großer Zielpatientenpopulationen in dieser Region zurückzuführen. Der asiatisch-pazifische Raum hingegen wird im Prognosezeitraum voraussichtlich die höchste Wachstumsrate aufweisen, da sich die Gesundheitsinfrastruktur verbessert und Neurochirurgen in dieser Region zunehmend über die Vorteile interventioneller neurologischer Geräte im Bilde sind.
Der Länderabschnitt des Marktberichts für interventionelle neurologische Geräte enthält auch Angaben zu einzelnen marktbeeinflussenden Faktoren und Änderungen der Regulierung auf dem Inlandsmarkt, die sich auf die aktuellen und zukünftigen Trends des Marktes auswirken. Datenpunkte wie Verbrauchsmengen, Produktionsstandorte und -mengen, Import-Export-Analyse, Preistrendanalyse, Rohstoffkosten, Downstream- und Upstream-Wertschöpfungskettenanalyse sind einige der wichtigsten Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Bei der Prognoseanalyse der Länderdaten werden auch die Präsenz und Verfügbarkeit globaler Marken und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen inländischer Zölle und Handelsrouten berücksichtigt.
Wachstum der Gesundheitsinfrastruktur, installierte Basis und Durchdringung mit neuen Technologien
Der Markt für interventionelle neurologische Geräte bietet Ihnen außerdem eine detaillierte Marktanalyse für jedes Land, das Wachstum der Gesundheitsausgaben für Investitionsgüter, die installierte Basis verschiedener Arten von Produkten für den Markt für interventionelle neurologische Geräte, die Auswirkungen der Technologie anhand von Lebenslinienkurven und Änderungen der regulatorischen Szenarien im Gesundheitswesen und deren Auswirkungen auf den Markt für interventionelle neurologische Geräte. Die Daten sind für den historischen Zeitraum 2010 bis 2020 verfügbar.
Wettbewerbsumfeld und globale Analyse der Marktanteile für interventionelle neurologische Geräte
Die Wettbewerbslandschaft auf dem Markt für interventionelle neurologische Geräte liefert Details nach Wettbewerbern. Die enthaltenen Details sind Unternehmensübersicht, Unternehmensfinanzen, erzielter Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -einrichtungen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang, Anwendungsdominanz. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus der Unternehmen in Bezug auf den Markt für interventionelle neurologische Geräte.
Zu den wichtigsten Akteuren auf dem Markt für interventionelle neurologische Geräte zählen unter anderem Johnson & Johnson Private Limited, Abbott, Boston Scientific Corporation, Stryker, Medtronic, Terumo Corporation, Penumbra, Inc., MicroPort Scientific Corporation, Merit Medical Systems, WL Gore & Associates, Inc., B. Braun Melsungen AG, UreSil, LLC, Medikit co., ltd., Bayer AG, Acandis GmbH, Biosensors International Group, Ltd., Medical Devices Business Services, Inc., Surtex Instruments Limited, Cook, LivaNova PLC und Integra LifeSciences.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.