Asia Pacific Dermatology Diagnostic Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
978.30 Million
USD
2,664.00 Million
2024
2032
USD
978.30 Million
USD
2,664.00 Million
2024
2032
| 2025 –2032 | |
| USD 978.30 Million | |
| USD 2,664.00 Million | |
|
|
|
|
Marktsegmentierung für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum nach Diagnosegerät (Bildgebungsgerät, Dermatoskop, Mikroskop), Produkttyp (Dermatoskope, Bildgebungsgeräte, Mikroskope und Trichoskope, Biopsiegeräte, Sonstiges), Behandlungsgerät (Elektrochirurgie, Kryotherapie, Laser), Anwendung (Hautkrebs, Akne, Psoriasis, Hautverjüngung, Warzen, Sonstiges), Endbenutzer (Krankenhäuser, Kliniken, Sonstiges) – Branchentrends und Prognose bis 2032
Dermatologische Diagnosegeräte Marktgröße
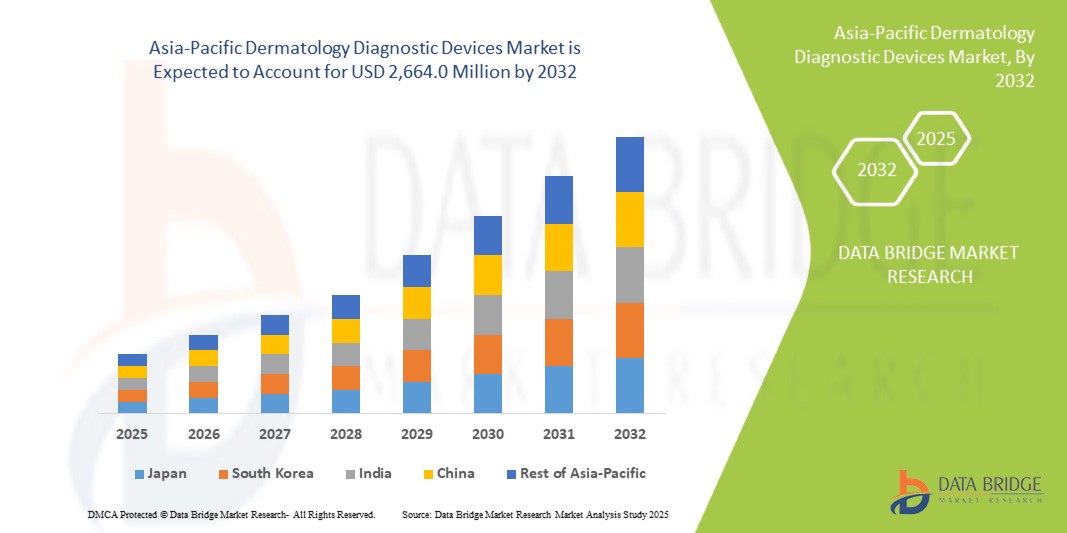
- Der Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum wurde im Jahr 2024 auf 978,3 Millionen US-Dollar geschätzt und soll bis 2032 2.664,0 Millionen US-Dollar erreichen, bei einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 13,34 % im Prognosezeitraum.
- Das Wachstum des Marktes für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum wird von mehreren entscheidenden Faktoren getrieben. Einer der Haupttreiber ist die zunehmende Zahl von Hautkrankheiten wie Hautkrebs, Akne und Schuppenflechte, die zu einer erhöhten Nachfrage nach fortschrittlichen dermatologischen Diagnosegeräten geführt hat. Darüber hinaus hat das steigende Bewusstsein für Hautgesundheit, das steigende verfügbare Einkommen und der Zugang zur Gesundheitsversorgung den Einsatz von Diagnoseinstrumenten zur Früherkennung und Behandlung gefördert.
Marktanalyse für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum
- Der Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum verzeichnet ein starkes Wachstum aufgrund des zunehmenden Bewusstseins für Hautgesundheit, der zunehmenden Prävalenz dermatologischer Erkrankungen und der steigenden Nachfrage nach frühzeitiger Diagnose und Behandlung. Dermatologische Diagnosegeräte sind wichtige Instrumente zur Erkennung und Überwachung von Hauterkrankungen wie Hautkrebs, Akne, Schuppenflechte, Ekzemen und anderen Hautinfektionen. Der Markt umfasst eine breite Palette von Geräten, darunter Bildgebungssysteme, Diagnosegeräte und Handgeräte, die zunehmend in klinischen Einrichtungen, dermatologischen Kliniken und Krankenhäusern in der gesamten Region eingesetzt werden.
- China wird im Prognosezeitraum voraussichtlich das am schnellsten wachsende Land im Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum sein. Dies ist auf den rasanten Ausbau der Gesundheitsinfrastruktur, das zunehmende Bewusstsein für Hautkrankheiten und die steigende Nachfrage nach Frühdiagnosen dermatologischer Erkrankungen zurückzuführen. Die zunehmende Nutzung von Telemedizin und Teledermatologie sowie steigende Investitionen im Gesundheitswesen fördern das Marktwachstum in Ländern wie China, Indien und Japan.
- China wird voraussichtlich den Markt für dermatologische Diagnosegeräte mit einem Anteil von 36,4 % dominieren. Dies ist auf die gut ausgebaute Gesundheitsinfrastruktur, die hohe Akzeptanz fortschrittlicher Diagnosetechnologien wie KI-gestützter Systeme und die Präsenz wichtiger Marktteilnehmer zurückzuführen. Der zunehmende Fokus der Region auf Hautkrebsprävention und -behandlung sowie günstige Erstattungsrichtlinien untermauern die starke Marktposition des Landes.
- Das Dermatoskop-Segment wird voraussichtlich mit einem Marktanteil von 34,5 % den Markt dominieren. Dies ist auf die weit verbreitete klinische Anwendung, die Kosteneffizienz und die Vertrautheit der Dermatologen mit den Verfahren zurückzuführen. Trotz des Aufkommens fortschrittlicherer Technologien wie der optischen Kohärenztomographie und der Konfokalmikroskopie bleiben Dermatoskope aufgrund ihrer Benutzerfreundlichkeit, Tragbarkeit und Effektivität bei der Diagnose von Hauterkrankungen wie Melanomen das Standardinstrument in vielen klinischen Umgebungen. Ihre langjährige Erfolgsgeschichte in der Früherkennung und ihre Fähigkeit, nicht-invasive Diagnostik anzubieten, stärken ihre marktführende Position weiter.
Berichtsumfang Marktsegmentierung für dermatologische Diagnosegeräte
|
Eigenschaften |
Wichtige Markteinblicke zu dermatologischen Diagnosegeräten |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Asien-Pazifik
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch Import-Export-Analysen, eine Übersicht über die Produktionskapazität, eine Analyse des Produktionsverbrauchs, eine Preistrendanalyse, ein Szenario des Klimawandels, eine Lieferkettenanalyse, eine Wertschöpfungskettenanalyse, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, eine PESTLE-Analyse, eine Porter-Analyse und regulatorische Rahmenbedingungen. |
Markttrends für dermatologische Diagnosegeräte
„Innovationen in der Diagnosetechnologie und der Wandel hin zur digitalen Gesundheitsintegration“
- Der Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum erlebt mit der Einführung modernster Technologien wie KI-gestützten Diagnosetools, hochauflösenden Bildgebungssystemen und Teledermatologie-Lösungen einen tiefgreifenden Wandel. Diese Innovationen verbessern die Präzision, Effizienz und Zugänglichkeit der Hautgesundheitsdiagnostik. Insbesondere KI-Algorithmen werden in Geräte integriert, um Erkrankungen wie Hautkrebs, Akne und Schuppenflechte schneller und genauer zu erkennen.
- Mit der zunehmenden Verbreitung digitaler Gesundheitslösungen zeichnet sich ein Trend zur Integration dermatologischer Diagnosegeräte in digitale Gesundheitsplattformen ab. Diese Plattformen bieten Echtzeitzugriff auf Diagnoseergebnisse und ermöglichen Patienten, ihre Hautgesundheit im Laufe der Zeit zu verfolgen. Telemedizin und mobile Gesundheits-Apps spielen eine immer wichtigere Rolle, insbesondere in abgelegenen und unterversorgten Gebieten, da sie dermatologische Beratungen und Diagnosen über virtuelle Plattformen anbieten.
- In China beispielsweise integriert das in Peking ansässige Startup DXY KI-gestützte Analysen mit Telemedizin-Plattformen, um die dermatologische Diagnostik zu revolutionieren. Das Unternehmen entwickelte eine KI-gestützte mobile App, mit der Nutzer Bilder ihrer Hauterkrankungen aufnehmen und sofort präzise Diagnosen erhalten können. Die App nutzt maschinelle Lernalgorithmen, um Bilder von Hautläsionen zu analysieren und so Einblicke in mögliche Erkrankungen wie Akne, Ekzeme und Hautkrebs zu geben.
- Teledermatologische Dienstleistungen breiten sich rasant aus, insbesondere in ländlichen und abgelegenen Gebieten, in denen der Zugang zu Dermatologen eingeschränkt ist. Dies bietet Herstellern von Diagnosegeräten die Möglichkeit, telemedizinische Tools anzubieten, mit denen Dermatologen Hautbilder aus der Ferne analysieren und Diagnosen stellen können. Dieser Trend dürfte sich insbesondere in Ländern mit großer ländlicher Bevölkerung wie Indien und Indonesien deutlich verstärken.
Marktdynamik für dermatologische Diagnosegeräte
Treiber
„Wachsendes Bewusstsein für Hautgesundheit und technologische Innovationen in der dermatologischen Diagnostik“
- TDer Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum wird maßgeblich durch die zunehmende Verbreitung von Hautkrankheiten wie Hautkrebs, Akne, Schuppenflechte und Ekzemen sowie das wachsende öffentliche Bewusstsein für die Bedeutung frühzeitiger Diagnose und Prävention vorangetrieben. Dieses wachsende Interesse an der Hautgesundheit, verbunden mit steigenden Gesundheitsausgaben, führt zu einer erhöhten Nachfrage nach fortschrittlichen Diagnoseinstrumenten, insbesondere solchen mit nicht-invasiver Echtzeit-Überwachung.
- Der Einsatz KI-gestützter Diagnosetechnologien wie maschinellem Lernen und Bilderkennungssystemen verändert die dermatologische Landschaft in der gesamten Region. Länder wie Japan, Südkorea und China sind Vorreiter bei der Einführung dieser Innovationen, die eine schnellere und präzisere Diagnose von Hauterkrankungen ermöglichen. KI-gestützte Tools können Bilder von Hautläsionen analysieren, frühe Anzeichen von Hautkrebs erkennen und den Verlauf chronischer Erkrankungen wie Akne und Schuppenflechte verfolgen. Diese Technologien helfen Dermatologen, eine bessere Versorgung zu gewährleisten und die Behandlungsergebnisse zu verbessern.
Zum Beispiel,
- Mindray Medical International Limited (China) brachte 2019 ein hochauflösendes Bildgebungssystem auf den Markt, das künstliche Intelligenz mit Hautdiagnostik kombiniert, insbesondere zur Früherkennung von Melanomen und anderen Hautkrebsarten. Dieses Gerät, das in Krankenhäusern und dermatologischen Kliniken in ganz China weit verbreitet ist, bietet eine höhere Genauigkeit bei der Identifizierung von Hautläsionen. Das innovative Produkt von Mindray revolutioniert die Art und Weise, wie Dermatologen Hautkrebs erkennen, ermöglicht ihnen frühzeitige Interventionen und reduziert das Risiko von Spätdiagnosen.
- Laut der Weltgesundheitsorganisation werden beispielsweise im asiatisch-pazifischen Raum jährlich über zwei Millionen neue Fälle von nicht-melanozytärem Hautkrebs diagnostiziert. Als Reaktion darauf ist der Markt für KI-gestützte dermatologische Diagnostik in China seit 2020 jährlich um über 25 % gewachsen. Plattformen wie Ping An Good Doctor und Alibaba Health nutzen KI-Algorithmen zur Unterstützung dermatologischer Untersuchungen. Darüber hinaus meldete das südkoreanische Gesundheitsministerium einen Anstieg der dermatologischen KI-Integration in Krankenhäusern um 40 % zwischen 2020 und 2023. Dies steigert die diagnostische Effizienz und verkürzt die Behandlungszeit für Patienten.
- Auch die Nachfrage der Verbraucher nach präventiver Dermatologie steigt. Immer mehr Menschen suchen nach Früherkennungsmethoden für Hautkrebs, insbesondere in sonnenreichen Regionen wie Australien und Südostasien. Moderne Bildgebungssysteme, digitale Dermatoskope und tragbare Diagnosegeräte ermöglichen es, die Gesundheit der Haut proaktiv zu überwachen und treiben so das Marktwachstum voran.
Gelegenheit
„Steigende Nachfrage nach nicht-invasiven, ethischen und personalisierten dermatologischen Lösungen“
- Die steigende Nachfrage nach nicht-invasiven, „Clean Label“- und ethisch produzierten dermatologischen Diagnosegeräten eröffnet Herstellern im asiatisch-pazifischen Raum erhebliche Chancen. Verbraucher suchen zunehmend nach Diagnoselösungen, die Transparenz, Sicherheit und hochwertige, wissenschaftlich fundierte Technologie in den Vordergrund stellen. Insbesondere steigt die Nachfrage nach Geräten, die keine invasiven Eingriffe erfordern und einen ethischeren Ansatz für die Hautgesundheit ermöglichen. Dieser Trend wird durch das wachsende Gesundheitsbewusstsein und die Präferenz für Lösungen vorangetrieben, die den ethischen Werten der Region entsprechen, insbesondere in Ländern wie Japan, Südkorea und Australien.
- Personalisierte Dermatologie bietet eine weitere große Marktchance. Die Anwendung genetischer Profile, KI-basierter Hautgesundheitsanalysen und metabolischer Erkenntnisse verändert die Diagnose und Behandlung dermatologischer Erkrankungen. Unternehmen bieten mittlerweile maßgeschneiderte Hautpflege- und Diagnosetools an, die auf individuellen genetischen und umweltbedingten Faktoren basieren. Die Integration KI-gestützter Diagnosegeräte, die Hautzustände analysieren und personalisierte Behandlungen empfehlen, gewinnt insbesondere in Hightech-Märkten wie Südkorea und Japan an Bedeutung.
Zum Beispiel,
- Im Jahr 2023 brachte Heine Optotechnik eine neue Produktlinie nicht-invasiver digitaler Dermatoskope auf den asiatisch-pazifischen Markt. Diese Geräte ermöglichen hochauflösende Echtzeit-Hautbilder mit integrierter KI für eine schnellere und präzisere Hautkrebserkennung. Das Unternehmen betonte die ethische Materialbeschaffung, nachhaltige Produktionspraktiken und Transparenz bei der Geräteentwicklung, was in Märkten wie Japan und Australien großen Anklang fand.
- Mit der Markteinführung wird die wachsende Nachfrage der Verbraucher nach ethischen, nicht-invasiven und wissenschaftlich validierten Diagnoseinstrumenten bedient und eine bedeutende Chance auf dem regionalen Markt für dermatologische Diagnosegeräte dargestellt.
Einschränkung/Herausforderung
„Regulierungskomplexität und Authentizitätsprobleme behindern das Marktwachstum“
- Der Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum steht aufgrund der Komplexität der Regulierung und unterschiedlicher Standards in den verschiedenen Ländern vor erheblichen Herausforderungen. Der regulatorische Rahmen für Medizinprodukte, einschließlich dermatologischer Diagnoseinstrumente, ist in der Region sehr unterschiedlich, was Hürden im Zulassungsprozess, bei Produktzertifizierungen und Kennzeichnungsvorschriften mit sich bringt. Länder wie China, Indien und Japan haben unterschiedliche Vorschriften, was zu Verzögerungen bei der Produkteinführung, erhöhten Compliance-Kosten und Schwierigkeiten bei der Vermarktung von Geräten führen kann, die den lokalen Standards entsprechen.
- Bedenken hinsichtlich der Echtheit und Wirksamkeit von Produkten stellen eine weitere Herausforderung im Markt für dermatologische Diagnosegeräte dar, insbesondere angesichts der zunehmenden Verbreitung gefälschter oder minderwertiger Produkte. Einige unregulierte oder minderwertige Geräte, oft importiert oder lokal hergestellt, erfüllen nicht die Sicherheits- und Leistungsstandards und schädigen das Vertrauen der Verbraucher in dermatologische Diagnoseprodukte. Dies ist insbesondere in Schwellenländern wie Indien und Südostasien ein Problem, wo es einen bedeutenden Markt für legale und illegale Geräte gibt.
- Da in der Region zunehmend Wert auf Transparenz, klinische Validierung und ethische Herstellung gelegt wird, stehen Hersteller dermatologischer Geräte zunehmend unter Druck, sicherzustellen, dass ihre Produkte klinisch validiert, von unabhängigen Stellen zertifiziert und internationalen Standards entsprechen. Dies erfordert erhebliche Investitionen in Tests, Zertifizierungen und kontinuierliche Überwachung, um die Produktsicherheit und -wirksamkeit zu gewährleisten. Dieser Trend zu höheren Qualitätsstandards ist zwar langfristig vorteilhaft, erhöht aber die Betriebskosten und kann die Entwicklungszeiten verlängern.
Zum Beispiel,
- Im Jahr 2022 führte die chinesische Nationale Medizinproduktebehörde (NMPA) eine umfassende Überprüfung und einen Rückruf mehrerer dermatologischer Diagnosegeräte durch, die den Sicherheitsstandards nicht entsprachen, insbesondere solcher zur Hautkrebserkennung. Auslöser für den Rückruf waren Bedenken hinsichtlich der Genauigkeit der Ergebnisse und ihrer Zuverlässigkeit unter realen Bedingungen.
- Dies führte nicht nur zu finanziellen Rückschlägen für die Hersteller, sondern auch zu einem Vertrauensverlust der Verbraucher in ähnliche Produkte. Der Schritt verdeutlichte die zunehmende regulatorische Kontrolle und die Herausforderungen, vor denen Unternehmen stehen, um die strengen Produktstandards in der Region einzuhalten.
Marktumfang für dermatologische Diagnosegeräte
Der Markt ist segmentiert auf der Grundlage von Diagnosegerät, Produkttyp, Behandlungsgerät, Anwendung und Endbenutzer
|
Segmentierung |
Untersegmentierung |
|
Nach Diagnosegerät |
|
|
Nach Produkttyp |
|
|
Nach Behandlungsgerät |
|
|
Nach Anwendung |
|
|
Nach Endbenutzer |
|
Im Jahr 2025 wird Hautkrebs voraussichtlich den Markt dominieren und den größten Anteil im Anwendungssegment haben.
Das Segment Hautkrebs wird voraussichtlich den Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum dominieren und im Jahr 2025 mit 32,22 % den größten Anteil ausmachen. Dies ist auf die steigende Zahl von Hautkrebserkrankungen zurückzuführen, insbesondere in Ländern mit hoher Sonneneinstrahlung wie Australien, Indien und Südostasien. Mit dem zunehmenden öffentlichen Bewusstsein für Hautkrebs steigt die Nachfrage nach nicht-invasiven, präzisen Diagnoseinstrumenten, die eine frühzeitige Erkennung und Behandlung ermöglichen. Geräte wie digitale Dermatoskope und KI-gestützte Bildgebungssysteme erfreuen sich zunehmender Beliebtheit in der Hautkrebserkennung, da sie hochauflösende, detaillierte Bilder von Hautläsionen liefern und potenziell krebsartige Wucherungen frühzeitig erkennen können. Diese Innovationen erfreuen sich bei Dermatologen und Gesundheitsdienstleistern aufgrund ihrer Effizienz, Präzision und Fähigkeit, schnellere Diagnosen zu ermöglichen, zunehmender Beliebtheit.
Es wird erwartet, dass die Elektrochirurgie im Prognosezeitraum den größten Anteil am Markt für Behandlungsgeräte ausmacht
Im Jahr 2025 wird das Segment Elektrochirurgie voraussichtlich den Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum dominieren und mit geschätzten 51,34 % den größten Marktanteil einnehmen. Dies ist auf die steigende Nachfrage nach präzisen und minimalinvasiven Verfahren in der dermatologischen Versorgung zurückzuführen. Diese Geräte werden häufig zur Entfernung von Hautläsionen, zur Behandlung von Warzen, Muttermalen und oberflächlichem Hautkrebs eingesetzt und bieten Vorteile wie reduzierte Blutungen, minimale Narbenbildung und schnellere Genesung. Die zunehmende Verbreitung dermatologischer Erkrankungen und das wachsende Bewusstsein für fortschrittliche Behandlungsmöglichkeiten fördern die Einführung elektrochirurgischer Technologien. Darüber hinaus erhöhen Fortschritte bei der Gerätesicherheit, Präzision und Integration mit anderen dermatologischen Instrumenten deren Effektivität und fördern das Marktwachstum in der gesamten Region.
Regionale Analyse des Marktes für dermatologische Diagnosegeräte
„China ist das dominierende Land auf dem Markt für dermatologische Diagnosegeräte“
- Prognosen zufolge wird China im Jahr 2025 den Markt für dermatologische Diagnosegeräte im asiatisch-pazifischen Raum anführen und den größten Marktanteil erobern. Grund dafür sind die wachsende Gesundheitsinfrastruktur, die zunehmende Belastung durch Hautkrankheiten und die steigenden Investitionen in dermatologische Forschung und Technologie.
- Das Land verzeichnet einen deutlichen Anstieg dermatologischer Erkrankungen wie Akne, Ekzemen, Schuppenflechte und Hautkrebs. Ursachen hierfür sind Umweltverschmutzung, veränderte Lebensstile und höhere UV-Belastung. Dies führt zu einer starken Nachfrage nach modernen Diagnoseinstrumenten, die eine frühzeitige Erkennung und wirksame Behandlung von Hauterkrankungen ermöglichen.
- Staatlich geförderte Gesundheitsinitiativen, darunter der Ausbau dermatologischer Abteilungen in öffentlichen Krankenhäusern und die Erhöhung der Mittel für Hautkrebs-Screening-Programme, tragen zu einer breiteren Akzeptanz dermatologischer Diagnosegeräte bei. Darüber hinaus beschleunigt Chinas proaktive Haltung bei der Integration von Technologie in das Gesundheitswesen die Verbreitung digitaler Dermatoskope, KI-gestützter Hautanalysesysteme und nicht-invasiver Bildgebungsverfahren.
- China entwickelt sich zudem zu einem Zentrum für die Herstellung und Innovation medizinischer und diagnostischer Geräte und bietet kostengünstige Lösungen, die rasch die behördlichen Genehmigungen erhalten und sowohl im Inland als auch in den Nachbarländern übernommen werden.
- Die wachsende Beliebtheit von Teledermatologie und mobilen Gesundheitsplattformen verbessert den Zugang zur dermatologischen Versorgung, insbesondere in ländlichen und halbstädtischen Regionen. Diese digitalen Lösungen, oft mit diagnostischen Bildgebungsfunktionen ausgestattet, erweitern die Reichweite der dermatologischen Diagnostik und fördern die Patientenbeteiligung am Hautgesundheitsmanagement.
- Dank seiner großen Bevölkerungsbasis, der schnellen Einführung neuer Technologien und strategischer staatlicher Unterstützung gibt China weiterhin das Tempo des Marktwachstums im Segment dermatologischer Diagnosegeräte im asiatisch-pazifischen Raum vor.
„Indien wird voraussichtlich die höchste Wachstumsrate verzeichnen“
- In Indien ist ein deutlicher Anstieg dermatologischer Erkrankungen wie Akne, Pilzinfektionen, Ekzemen und Hautkrebs zu verzeichnen. Ursachen hierfür sind die Umweltverschmutzung in den Städten, steigender Stress, ungesunde Lebensgewohnheiten und übermäßige Sonneneinstrahlung. Dieser Anstieg hautbezogener Probleme führt zu einer steigenden Nachfrage nach Frühdiagnosen und effektiven Technologien zur Hautbeurteilung.
- Die indische Regierung fördert die Früherkennung von Krankheiten und einen erschwinglichen Zugang zur Gesundheitsversorgung durch Initiativen wie Ayushman Bharat und die Erhöhung der Mittel für medizinische Versorgungszentren – viele davon bieten mittlerweile auch dermatologische Leistungen an. Diese Maßnahmen unterstützen den Ausbau der dermatologischen Infrastruktur und fördern den Einsatz von Diagnosegeräten im öffentlichen und privaten Gesundheitswesen.
- Mit dem wachsenden Trend zu personalisierter und präventiver Gesundheitsversorgung entscheiden sich indische Verbraucher zunehmend für Frühdiagnosen und dermatologische Untersuchungen, insbesondere in städtischen Regionen. Dieser Bedarf wird durch die Integration fortschrittlicher Tools wie digitaler Dermatoskope, optischer Bildgebungssysteme und KI-gestützter Hautanalyseplattformen in Kliniken und Wellnesszentren gedeckt.
- Indiens florierende Telemedizinbranche und das digitale Gesundheitsökosystem tragen zusätzlich zum Zugang zu dermatologischer Diagnostik in ländlichen und halbstädtischen Gebieten bei. Der Einsatz mobiler Diagnosegeräte und cloudbasierter Hautanalysetools verbessert die Früherkennung und Fernkonsultationen und schließt so die Lücke zwischen Dermatologen und Patienten im Land.
Marktanteil dermatologischer Diagnosegeräte
Die Wettbewerbslandschaft des Marktes liefert detaillierte Informationen zu den einzelnen Wettbewerbern. Zu den Details gehören Unternehmensübersicht, Unternehmensfinanzen, Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, globale Präsenz, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben genannten Datenpunkte beziehen sich ausschließlich auf die Marktausrichtung der Unternehmen.
Die wichtigsten Marktführer auf dem Markt sind:
- Canfield Scientific (USA)
- Heine Optotechnik (Deutschland)
- Olympus Corporation (Japan)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- FUJIFILM Holdings Corporation (Japan)
- Canon Medical Systems Corporation (Japan)
- Hitachi, Ltd. (Tokio, Japan)
- Samsung Medison Co., Ltd. (Südkorea)
- Opto Circuits (India) Ltd. (Indien)
- Arkray, Inc. (Japan)
- Mindray Medical International Limited (China)
Neueste Entwicklungen auf dem Markt für dermatologische Diagnosegeräte
- Im Februar 2025 ging Canfield Scientific eine Partnerschaft mit einer großen dermatologischen Klinikkette in Südkorea ein, um seine VISIA-Hautanalysesysteme an mehreren Standorten einzusetzen. Dieser Schritt verbessert die diagnostische Präzision sowohl in der ästhetischen als auch in der medizinischen Dermatologie und trägt der steigenden Nachfrage der Verbraucher nach KI-integrierten Hautbewertungstools Rechnung.
- Im Januar 2025 erweiterte FotoFinder seine KI-gestützten Dermatoskopiegeräte durch strategische Partnerschaften mit lokalen Distributoren nach Japan und Australien. Die Initiative zielt darauf ab, die Früherkennung von Melanomen und die diagnostische Genauigkeit für Hochrisikogruppen in diesen Regionen zu verbessern.
- Im März 2025 erhielt DermaSensor in Singapur die behördliche Zulassung für sein nicht-invasives Handgerät mit optischer Spektroskopie, das Hausärzte bei der Erkennung von Hautkrebs unterstützt. Diese Zulassung ermöglicht eine bessere und zeitnahe Hautdiagnostik in ganz Südostasien.
- Im April 2025 kooperierte HEINE Optotechnik mit Teledermatologie-Startups in Indien, um seine Dermatoskope in Smartphone-basierte Diagnoseplattformen zu integrieren. Ziel dieser Partnerschaft ist es, Versorgungslücken in ländlichen Gebieten zu schließen und die Früherkennung von Hautkrankheiten in unterversorgten Gebieten zu fördern.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.