Asia Pacific Bladder Cancer Diagnostics Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
360.00 Million
USD
646.84 Million
2024
2032
USD
360.00 Million
USD
646.84 Million
2024
2032
| 2025 –2032 | |
| USD 360.00 Million | |
| USD 646.84 Million | |
|
|
|
|
Marktsegmentierung für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum nach Testtyp (Zystoskopie, Urinlabortest, Biopsie, bildgebendes Verfahren und andere), Stadien (Stadium I, Stadium II, Stadium III und Stadium IV), Krebsart (Urinkarzinom der Blase, Plattenepithelkarzinom der Blase und andere Krebsarten), Endverbraucher (Krankenhaus, Zentren für diagnostische Bildgebung, Krebsforschungsinstitute, unabhängige Diagnoselabore und verbundene Labore), Vertriebskanal (Direktausschreibung und Einzelhandelsverkauf) – Branchentrends und Prognose bis 2032
Marktgröße für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
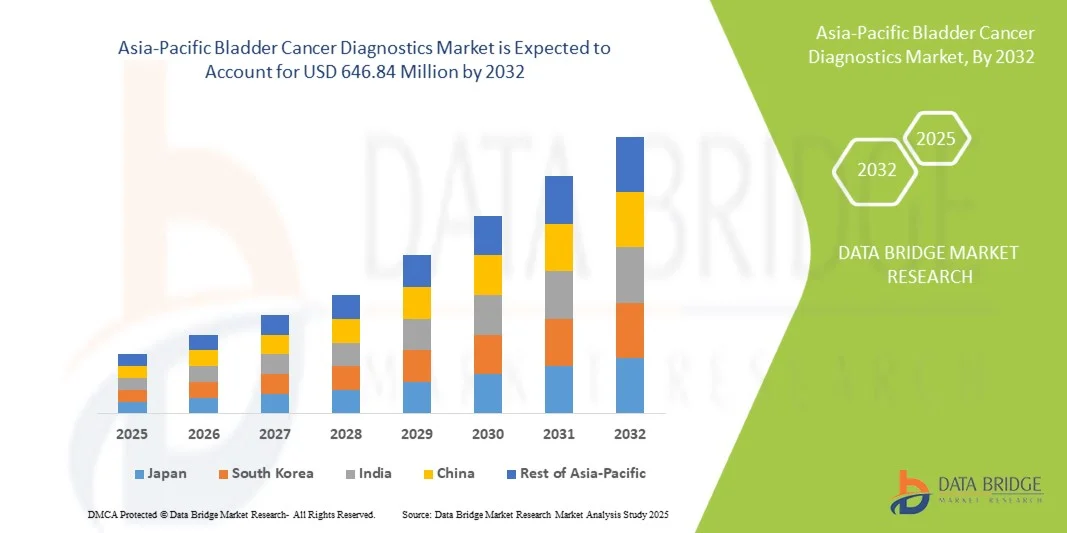
- Der Markt für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum hatte im Jahr 2024 ein Volumen von 360,00 Millionen US-Dollar und dürfte bis 2032 einen Wert von 646,84 Millionen US-Dollar erreichen , bei einer CAGR von 7,6 % im Prognosezeitraum.
- Das Marktwachstum wird maßgeblich durch die steigende Zahl von Blasenkrebserkrankungen in der Region, Fortschritte in der Diagnosetechnologie und einen verstärkten Fokus auf Früherkennung und personalisierte Behandlung vorangetrieben, was zu verbesserten Patientenergebnissen sowohl im klinischen als auch im Forschungsumfeld führt.
- Darüber hinaus treiben Länder wie Japan und China mit ihrer hochentwickelten Gesundheitsinfrastruktur und dem zunehmenden Zugang zur Gesundheitsversorgung die Einführung fortschrittlicher Diagnoselösungen voran und etablieren die Blasenkrebsdiagnostik als wichtigen Bestandteil regionaler Gesundheitsstrategien. Diese konvergierenden Faktoren beschleunigen die Verbreitung diagnostischer Instrumente und fördern damit das Wachstum der Branche erheblich.
Marktanalyse für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
- Die Diagnostik von Blasenkrebs, die Tests wie Zystoskopie, Urinlabortests, Biopsie und bildgebende Verfahren umfasst, wird zu einem unverzichtbaren Instrument für die Früherkennung und Behandlung von Blasenkrebs sowohl im klinischen als auch im Forschungsumfeld, da sie die diagnostische Genauigkeit verbessert, eine personalisierte Behandlung ermöglicht und die kontinuierliche Patientenüberwachung unterstützt.
- Die steigende Nachfrage nach Blasenkrebsdiagnostik ist vor allem auf die zunehmende Verbreitung von Blasenkrebs im asiatisch-pazifischen Raum, das wachsende Bewusstsein für die Vorteile einer Früherkennung und den technologischen Fortschritt bei nicht-invasiven und molekularen Diagnoseinstrumenten zurückzuführen.
- Japan dominierte den Markt für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum mit dem größten Umsatzanteil von 29,9 % im Jahr 2024, unterstützt durch eine hochentwickelte Gesundheitsinfrastruktur, steigende Gesundheitsausgaben und die Präsenz wichtiger Diagnostikunternehmen, die sich auf hochpräzise Technologien konzentrieren
- Indien dürfte im Prognosezeitraum der am schnellsten wachsende Markt sein, da das Gesundheitsbewusstsein steigt, die Diagnosemöglichkeiten verbessert werden und die Regierung verstärkt Krebsvorsorgeprogramme einführt.
- Das Segment Zystoskopie dominierte den Markt für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum mit einem Marktanteil von 42,6 % im Jahr 2024, was auf seine hohe diagnostische Genauigkeit, die breite klinische Akzeptanz und die Fähigkeit zurückzuführen ist, Blasenkrebs im Frühstadium effektiv zu erkennen.
Berichtsumfang und Marktsegmentierung für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
|
Eigenschaften |
Wichtige Markteinblicke zur Blasenkrebsdiagnostik im asiatisch-pazifischen Raum |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Asien-Pazifik
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Preisanalysen, Markenanteilsanalysen, Verbraucherumfragen, demografische Analysen, Lieferkettenanalysen, Wertschöpfungskettenanalysen, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, PESTLE-Analysen, Porter-Analysen und regulatorische Rahmenbedingungen. |
Markttrends für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
Fortschritte bei nichtinvasiven und molekularen Diagnosetechnologien
- Ein bedeutender und sich beschleunigender Trend auf dem Markt für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum ist die zunehmende Einführung nicht-invasiver Urintests und molekularer Diagnoseinstrumente, die die Früherkennung verbessern und den Patientenkomfort erhöhen.
- Beispielsweise ermöglicht der UroVysion FISH-Test die Erkennung von Chromosomenanomalien in Urinproben und reduziert so die Abhängigkeit von invasiven Zystoskopieverfahren.
- Molekulare Tests ermöglichen die präzise Identifizierung von Krebssubtypen, die Erstellung personalisierter Behandlungspläne und die Echtzeitüberwachung des Krankheitsverlaufs
- Die Integration dieser fortschrittlichen Diagnostik in Krankenhausinformationssysteme ermöglicht es Klinikern, Patientendaten effizient zu verfolgen und die Entscheidungsfindung für Behandlungsstrategien zu unterstützen
- Dieser Trend zu präziseren, patientenfreundlicheren und technologisch fortschrittlicheren Diagnoselösungen verändert die klinischen Erwartungen und treibt die Nachfrage nach Instrumenten zur Früherkennung an
- Die Nachfrage nach nicht-invasiven und molekularen Diagnosetests steigt in Krankenhäusern, diagnostischen Bildgebungszentren und unabhängigen Laboren rasant an, da für Gesundheitsdienstleister Effizienz und Patientencompliance im Vordergrund stehen.
Marktdynamik für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
Treiber
Steigende Prävalenz von Blasenkrebs und Fokus auf Früherkennung
- Die steigende Zahl von Blasenkrebserkrankungen in den Ländern des asiatisch-pazifischen Raums sowie das gestiegene Bewusstsein für die Vorteile einer Früherkennung sind ein wichtiger Treiber für das Marktwachstum.
- Japan hat beispielsweise landesweite Screening-Programme zur Früherkennung von Blasenkrebs eingeführt und die Einführung fortschrittlicher Diagnosetests gefördert.
- Früherkennung ermöglicht rechtzeitige Behandlungsmaßnahmen, senkt die Behandlungskosten und verbessert die Überlebensraten der Patienten. Steigende Gesundheitsausgaben, der Ausbau der diagnostischen Infrastruktur und ein verbesserter Zugang zur Gesundheitsversorgung in Schwellenländern führen zu einer zunehmenden Nutzung diagnostischer Instrumente.
- Krankenhäuser und Diagnosezentren setzen zunehmend auf fortschrittliche Technologien wie Zystoskopie, Urintests und bildgebende Verfahren, um der steigenden Nachfrage gerecht zu werden und die Patientenversorgung zu verbessern.
- Steigende Investitionen privater Gesundheitsdienstleister und Diagnostikunternehmen im asiatisch-pazifischen Raum beschleunigen die Verfügbarkeit fortschrittlicher Diagnoseplattformen
- Die verstärkte Zusammenarbeit zwischen Forschungsinstituten und Gesundheitsdienstleistern treibt Innovationen bei Früherkennungsmethoden und personalisierter Diagnostik voran. Sensibilisierungskampagnen und Schulungsprogramme tragen zu einer höheren Patientenbeteiligung an Screening-Programmen bei und fördern so das Marktwachstum weiter.
Einschränkung/Herausforderung
Hohe Kosten für fortschrittliche Diagnostik und geringes Bewusstsein in Schwellenländern
- Die relativ hohen Kosten für fortschrittliche diagnostische Tests, wie etwa molekulare Tests und bildbasierte Diagnostik, stellen ein erhebliches Hindernis für die Einführung in preissensiblen Märkten dar.
- Beispielsweise sind hochentwickelte Urintests auf FISH-Basis für kleinere Kliniken oder Patienten in einkommensschwachen Regionen möglicherweise nicht erschwinglich, was die Marktdurchdringung einschränkt.
- Mangelndes Bewusstsein für die Früherkennung von Blasenkrebs und die Bedeutung regelmäßiger Vorsorgeuntersuchungen in ländlichen oder halbstädtischen Gebieten kann die Inanspruchnahme diagnostischer Lösungen einschränken
- Inkonsistente regulatorische Rahmenbedingungen in den Ländern des asiatisch-pazifischen Raums können Produktzulassungen verzögern und die rechtzeitige Einführung neuer Diagnosetechnologien behindern. Die begrenzte Verfügbarkeit von geschultem Personal für die Durchführung fortgeschrittener Diagnosetests in kleineren Krankenhäusern oder Laboren kann das Marktwachstum hemmen.
- Herausforderungen bei der Integration neuer Diagnosetechnologien in bestehende Arbeitsabläufe im Gesundheitswesen können die Akzeptanz verlangsamen und die betriebliche Effizienz beeinträchtigen. Sozioökonomische Unterschiede und der ungleiche Zugang zur Gesundheitsversorgung in bestimmten Regionen führen zu unterschiedlichen Akzeptanzraten diagnostischer Technologien.
- Die Bewältigung dieser Herausforderungen durch staatliche Subventionen, Schulungsprogramme und kostengünstige Diagnoselösungen wird für ein nachhaltiges Marktwachstum von entscheidender Bedeutung sein.
Marktumfang für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
Der Markt ist nach Testtyp, Stadium, Krebsart, Endbenutzer und Vertriebskanal segmentiert.
- Nach Testtyp
Der Markt für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum ist nach Testart in Zystoskopie, Urinlabortest, Biopsie, bildgebende Verfahren und weitere unterteilt. Das Zystoskopie-Segment dominierte den Markt mit dem größten Umsatzanteil von 42,6 % im Jahr 2024 aufgrund seiner hohen diagnostischen Genauigkeit und weit verbreiteten klinischen Anwendung. Die Zystoskopie ermöglicht eine direkte Visualisierung des Blaseninneren, wodurch Tumore frühzeitig erkannt und Biopsien präzise entnommen werden können. Krankenhäuser und spezialisierte Diagnosezentren bevorzugen die Zystoskopie aufgrund ihrer Zuverlässigkeit und etablierten klinischen Protokolle. Das Segment profitiert von kontinuierlichen technologischen Verbesserungen wie hochauflösender Bildgebung und flexiblen Endoskopen, die den Patientenkomfort und die Verfahrenseffizienz erhöhen. Das zunehmende Vertrauen der Ärzte und das Bewusstsein der Patienten fördern ebenfalls die Akzeptanz der Zystoskopie als primäres Diagnoseinstrument. Darüber hinaus unterstützen die Erstattungsrichtlinien in Ländern wie Japan ihre weit verbreitete Anwendung und stärken ihre Dominanz.
Das Segment der Urinlabortests wird im Prognosezeitraum voraussichtlich das schnellste Wachstum verzeichnen, angetrieben durch die steigende Nachfrage nach nicht-invasiven, kostengünstigen Diagnoselösungen. Urinbasierte Tests bieten eine einfache Probenentnahme, Wiederholbarkeit für die Überwachung und eignen sich für Früherkennungsprogramme. Technologische Fortschritte wie Biomarker- Panels und molekulare Tests erhöhen die Sensitivität und Spezifität dieser Tests. Dieses Segment gewinnt insbesondere in Schwellenländern an Bedeutung, in denen der Zugang zu modernen Krankenhauseinrichtungen eingeschränkt ist. Der nicht-invasive Ansatz fördert die Patienten-Compliance und unterstützt groß angelegte Screening-Initiativen.
- Nach Etappen
Basierend auf dem Krankheitsstadium ist der Markt in Stadium I, Stadium II, Stadium III und Stadium IV segmentiert. Das Segment Stadium I dominierte den Markt mit einem Anteil von 36,5 % im Jahr 2024, da eine frühzeitige Erkennung für eine bessere Prognose und Überlebensraten entscheidend ist. Fortgeschrittene Diagnostik, einschließlich Zystoskopie und Urinlabortests, konzentriert sich in erster Linie auf die Identifizierung von Tumoren im Stadium I, um schnelle Behandlungsinterventionen zu ermöglichen. Kliniker priorisieren die Erkennung von Tumoren im Stadium I, da sie minimalinvasive Behandlungen ermöglicht und die langfristigen Gesundheitskosten senkt. Die Frühdiagnostik wird zunehmend in Krankenhausinformationssysteme integriert, um Patienten zu verfolgen und eine personalisierte Versorgung zu gewährleisten. Sensibilisierungskampagnen und staatlich geförderte Screening-Programme tragen ebenfalls zum Wachstum der Tests im Stadium I bei. Verbesserte Zugänglichkeit und das Vertrauen der Kliniker stärken ihre dominante Stellung.
Das Segment Stadium II wird im Prognosezeitraum voraussichtlich das schnellste Wachstum verzeichnen, angetrieben durch eine verbesserte diagnostische Infrastruktur und die zunehmende Nutzung molekularer und bildgebender Verfahren zur Erkennung von Erkrankungen im mittleren Stadium. Die Erkennung von Stadium II ermöglicht ein rechtzeitiges Eingreifen und verhindert oft ein Fortschreiten der Erkrankung in fortgeschrittene Stadien. Investitionen in Krankenhauslabore und private Diagnosezentren verbessern die Diagnosemöglichkeiten im Stadium II. Die steigende Prävalenz von Blasenkrebs in Ländern wie Indien und China treibt die Nachfrage nach auf das Stadium II ausgerichteter Diagnostik an.
- Nach Krebsart
Der Markt ist nach Krebsart segmentiert in Urothelkarzinom der Blase, Plattenepithelkarzinom der Blase und weitere Krebsarten. Urothelkarzinom der Blase dominierte den Markt mit einem Anteil von 68,2 % im Jahr 2024, da es die häufigste Blasenkrebsart im asiatisch-pazifischen Raum darstellt. Speziell auf Urothelkarzinome zugeschnittene Diagnostikverfahren, darunter Zystoskopie und Urinzytologie, sind weit verbreitet und in klinische Standardprotokolle integriert. Hohe Prävalenzraten und etablierte Behandlungspfade unterstützen die anhaltende Akzeptanz dieser Diagnosemethoden. Das Segment profitiert von kontinuierlichen technologischen Verbesserungen und der Vertrautheit der Ärzte. Aufklärungskampagnen zum Urothelkarzinom untermauern seine Dominanz weiter.
Das Segment Plattenepithelkarzinom der Blase dürfte im Prognosezeitraum aufgrund steigender Erkennungsraten und technologischer Fortschritte in der Molekulardiagnostik das schnellste Wachstum verzeichnen. Obwohl seltener, erfordert das Plattenepithelkarzinom spezielle Tests zur Früherkennung und effektiven Behandlungsplanung. Verbesserte Laborinfrastruktur und neue Biomarkerforschung treiben das Wachstum in diesem Segment voran. Länder mit steigender Bilharziose-Prävalenz tragen ebenfalls zu einem verstärkten Fokus auf die Plattenepithelkarzinom-Diagnostik bei.
- Nach Endbenutzer
Auf Basis der Endnutzer ist der Markt in Krankenhäuser, Zentren für diagnostische Bildgebung, Krebsforschungsinstitute, unabhängige Diagnoselabore und zugehörige Labore segmentiert. Das Krankenhaussegment dominierte den Markt mit einem Anteil von 51,4 % im Jahr 2024, da Krankenhäuser über fortschrittliche Diagnoseinstrumente, qualifiziertes Klinikpersonal und integrierte Patientenversorgungssysteme verfügen. Krankenhäuser führen Zystoskopie, Bildgebung und Biopsie häufig intern durch und bieten so umfassende Diagnoselösungen. Steigende Gesundheitsausgaben und die Präferenz der Patienten für krankenhausbasierte Diagnostik tragen zu dieser Dominanz bei. Krankenhäuser fungieren zudem als primäre Überweisungszentren und festigen so ihren Marktanteil weiter. Die Einführung von Krankenhausinformationssystemen und elektronischen Patientenakten ermöglicht ein besseres Workflow-Management und eine bessere Patientenverfolgung.
Das Segment der unabhängigen Diagnoselabore dürfte im Prognosezeitraum das schnellste Wachstum verzeichnen, angetrieben durch den zunehmenden Zugang zu erschwinglichen und spezialisierten Diagnosedienstleistungen. Diese Labore richten sich an die Bevölkerung in Vorstädten und ländlichen Gebieten und bieten Urin- und Molekulartests mit kürzeren Bearbeitungszeiten an. Kooperationen mit Krankenhäusern und staatlichen Screening-Programmen stärken ihre Marktpräsenz. Steigendes Gesundheitsbewusstsein und die zunehmende Präferenz der Patienten für günstige Testorte treiben das Wachstum des Segments voran.
- Nach Vertriebskanal
Der Markt wird nach Vertriebskanälen in Direktausschreibungen und Einzelhandelsverkäufe unterteilt. Das Segment Direktausschreibungen dominierte den Markt mit einem Anteil von 59,6 % im Jahr 2024, unterstützt durch Großbestellungen von Krankenhäusern, Regierungsinitiativen und großen Diagnosezentren. Direktausschreibungsvereinbarungen gewährleisten eine zuverlässige Versorgung mit Diagnosekits und -geräten zu ausgehandelten Preisen. Dieser Kanal wird bevorzugt für groß angelegte Krebsvorsorgeprogramme und hochvolumige Diagnoseeinrichtungen eingesetzt. Die institutionelle Akzeptanz sorgt für eine konstante Nachfrage und stärkt die Marktposition dieses Segments. Günstige Beschaffungsrichtlinien und langfristige Verträge tragen zu seiner Dominanz bei.
Der Einzelhandel wird im Prognosezeitraum voraussichtlich das schnellste Wachstum verzeichnen, angetrieben durch die steigende Nachfrage nach Urintests für zu Hause und Point-of-Care-Diagnostik. Das wachsende Bewusstsein für Selbsttests und präventive Gesundheitsfürsorge fördert die Akzeptanz im Einzelhandel. Komfort, einfacher Zugang und zunehmende Online-Vertriebskanäle fördern das Segmentwachstum. Schwellenländer mit wachsender Mittelschicht bieten zusätzliche Möglichkeiten zur Ausweitung des Einzelhandelsumsatzes.
Regionale Analyse des Marktes für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
- Japan dominierte den Markt für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum mit dem größten Umsatzanteil von 29,9 % im Jahr 2024, unterstützt durch eine hochentwickelte Gesundheitsinfrastruktur, steigende Gesundheitsausgaben und die Präsenz wichtiger Diagnostikunternehmen, die sich auf hochpräzise Technologien konzentrieren
- Patienten und Gesundheitsdienstleister in Japan legen zunehmend Wert auf Früherkennung, genaue Diagnostik und personalisierte Behandlungspläne, was die Nachfrage nach Zystoskopie, Urinuntersuchungen, Biopsien und bildgebenden Verfahren ankurbelt
- Diese weitverbreitete Akzeptanz wird durch staatliche Krebsvorsorgeprogramme, ein starkes Bewusstsein der Kliniker und technologische Innovationen bei Diagnoseplattformen weiter unterstützt, wodurch sich Japan als führender Markt für Blasenkrebsdiagnostik in der Region etabliert hat.
Einblicke in den japanischen Markt für Blasenkrebsdiagnostik
Der japanische Markt für Blasenkrebsdiagnostik ist der größte im asiatisch-pazifischen Raum und erzielte 2024 einen signifikanten Umsatzanteil von 29,9 %. Dieser wird durch eine fortschrittliche Gesundheitsinfrastruktur, hohe Gesundheitsausgaben und den Fokus auf die Krebsfrüherkennung vorangetrieben. Fortschrittliche Diagnoseverfahren wie Zystoskopie, Urintests, Biopsie und bildgebende Verfahren sind in Krankenhäusern und spezialisierten Diagnosezentren weit verbreitet. Landesweite Screening-Programme und staatliche Initiativen fördern Routineuntersuchungen, erhöhen die Patientenbeteiligung und die Früherkennungsraten. Die Integration der Diagnostik in Krankenhausinformationssysteme und personalisierte Behandlungsprotokolle verbessert die klinische Effizienz und die Patientenergebnisse. Technologische Fortschritte, darunter hochauflösende Bildgebung und molekulare Tests, unterstützen eine präzise und zeitnahe Diagnose. Das Bewusstsein und die Vorliebe der Ärzte für Präzisionsdiagnostik stärken Japans Marktdominanz zusätzlich.
Markteinblick in die Blasenkrebsdiagnostik in Indien
Der indische Markt für Blasenkrebsdiagnostik ist der am schnellsten wachsende im asiatisch-pazifischen Raum. Begünstigt wird dies durch die schnelle Urbanisierung, das zunehmende Bewusstsein für Blasenkrebs und die wachsende Gesundheitsinfrastruktur. Die wachsende Mittelschicht und die zunehmend erschwinglichere Gesundheitsversorgung treiben die Nachfrage nach Früherkennung und präziser Diagnostik. Krankenhäuser, unabhängige Diagnoselabore und Bildgebungszentren setzen zunehmend Zystoskopie, Urintests, Biopsien und bildgebende Verfahren ein, um die Patientennachfrage zu decken. Regierungsinitiativen zur Förderung von Krebsvorsorge und digitalen Gesundheitsprogrammen verbessern den Zugang zu Diagnostik in städtischen und halbstädtischen Gebieten. Kostengünstige Diagnoselösungen und die Präsenz privater Gesundheitsdienstleister beschleunigen die Marktdurchdringung. Darüber hinaus fördern das gestiegene Patientenbewusstsein und die steigende Prävalenz von Blasenkrebs eine rechtzeitige Diagnose und Behandlung.
Markteinblick in die Blasenkrebsdiagnostik in China
Der chinesische Markt für Blasenkrebsdiagnostik verzeichnet ein starkes Wachstum, das durch die zunehmende Prävalenz von Blasenkrebs, den Ausbau der Gesundheitsinfrastruktur und zunehmende staatliche Initiativen zur Krebsfrüherkennung vorangetrieben wird. Krankenhäuser und Diagnosezentren in städtischen Regionen setzen zunehmend auf moderne Verfahren wie Zystoskopie, Urinlabortests, bildgebende Verfahren und molekulare Tests. Die Integration der Diagnostik in elektronische Patientenakten und Krankenhausinformationssysteme verbessert die Arbeitsabläufe und die Patientenversorgung. Sensibilisierungskampagnen und Gesundheitserziehungsprogramme fördern frühzeitige Tests und Überwachung. Technologische Fortschritte, darunter KI-gestützte Bildgebung und nicht-invasive Urintests, verbessern die diagnostische Genauigkeit. Steigende Investitionen privater Diagnostikunternehmen und Forschungsinstitute treiben das Marktwachstum zusätzlich voran.
Markteinblick in die Blasenkrebsdiagnostik in Australien
Der australische Markt für Blasenkrebsdiagnostik wächst stetig. Dies ist auf einen hohen Gesundheitsstandard, die zunehmende staatliche Unterstützung von Krebsvorsorgeprogrammen und das steigende Bewusstsein der Patienten für die Vorteile der Früherkennung zurückzuführen. Krankenhäuser, Krebsforschungsinstitute und unabhängige Labore sind die Hauptanwender von Zystoskopie, Urinlabortests, Biopsien und bildgebender Diagnostik. Staatliche Initiativen zur Förderung routinemäßiger Vorsorgeuntersuchungen sowie Kooperationen mit privaten Gesundheitsdienstleistern verbessern die Zugänglichkeit und Akzeptanz. Fortschrittliche Diagnosetechnologien wie molekulare Tests und Bildgebungssysteme werden zunehmend in die klinische Praxis integriert. Die steigende Zahl von Blasenkrebserkrankungen bei älteren Menschen treibt die Nachfrage nach regelmäßigen Tests. Darüber hinaus unterstützen solide Erstattungsrahmen und hohe Gesundheitsausgaben das Marktwachstum.
Marktanteile der Blasenkrebsdiagnostik im asiatisch-pazifischen Raum
Die Blasenkrebsdiagnostikbranche im asiatisch-pazifischen Raum wird hauptsächlich von etablierten Unternehmen geführt, darunter:
- Astellas Pharma Inc. (Japan)
- Sysmex Corporation (Japan)
- FUJIFILM Corporation (Japan)
- Abbott (USA)
- F. Hoffmann-La Roche Ltd (Schweiz)
- GE HealthCare (USA)
- Siemens Healthineers AG (Deutschland)
- Koninklijke Philips NV, (Niederlande)
- Medtronic (Irland)
- Thermo Fisher Scientific Inc. (USA)
- Illumina, Inc. (USA)
- Bio-Rad Laboratories, Inc. (USA)
- PerkinElmer (USA)
- Agilent Technologies, Inc. (USA)
- QIAGEN (Deutschland)
- bioMérieux (Frankreich)
- Hologic, Inc. (USA)
- BD (USA)
- AbbVie Inc. (USA)
- Johnson & Johnson Services, Inc. (USA)
Was sind die jüngsten Entwicklungen auf dem Markt für Blasenkrebsdiagnostik im asiatisch-pazifischen Raum?
- Im Juli 2025 kündigte das Safdarjung Hospital in Neu-Delhi bedeutende Verbesserungen seiner urologischen Dienste an, darunter die Installation moderner medizinischer Geräte wie Indiens erstem Video-Urodynamisches Gerät mit Nahinfrarotspektroskopie. Diese Verbesserungen zielen darauf ab, die Diagnose- und Behandlungsmöglichkeiten für Blasenkrebspatienten zu verbessern und insbesondere wirtschaftlich benachteiligten Menschen zu helfen.
- Im Juni 2025 startete in Australien die ANZUP 1301-Studie, die größte randomisierte Studie zum Thema Blasenkrebs im letzten Jahrzehnt. An der Studie nehmen 501 Patienten an 17 Standorten teil. Ziel ist es, die Wirksamkeit einer neuen Kombinationsbehandlung bei risikoreichem, nicht-muskelinvasivem Blasenkrebs zu untersuchen und so die Notwendigkeit einer Blasenentfernung oder intensiver Therapien zu reduzieren.
- Im April 2025 brachte Craif, ein japanisches Startup aus der Universität Nagoya, den miSignal-Test auf den Markt – ein nicht-invasives, urinbasiertes Diagnosetool, das bis zu sieben Krebsarten, darunter auch Blasenkrebs, erkennen kann. Der Test nutzt microRNA-Biomarker und soll die Früherkennung verbessern und so den Bedarf an invasiven Verfahren wie der Zystoskopie reduzieren.
- Im März 2025 startete der indische Bundesstaat Telangana ein Pilotprogramm zur Integration künstlicher Intelligenz in Krebsvorsorgeprozesse. Der Schwerpunkt liegt dabei auf einer präziseren Früherkennung. Ziel der Initiative ist es, die Diagnosemöglichkeiten zu verbessern und Herausforderungen wie den Mangel an Radiologen zu bewältigen. Geplant ist die Ausweitung von KI-Tools an staatlichen medizinischen Hochschulen und die Einrichtung von Screening-Zentren auf Bezirksebene.
- Im Februar 2025 wurde in Australien ein neues Urin-Diagnosekit eingeführt, das eine nicht-invasive Methode zur Früherkennung von Blasenkrebs bietet. Dieses Kit soll die Frühdiagnose verbessern, unnötige Zystoskopien reduzieren und die Lebensqualität der Patienten durch Tests zu Hause verbessern.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.