Asia-Pacific Antiviral Drugs Market, By Indication (Influenza, Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), Respiratory Syncytial Virus, Herpes Simplex Virus, Human Cytomegalovirus (HCMV), Varicella-Zoster Virus (VZV), Hepatitis B Virus (HBV), Coronavirus Infection, and Others), Patient Type (Child, Adult, and Geriatric), Products (Oral, Topical, and Parenteral), Drug Type (Generic and Branded), End User (Hospitals, Clinics, Home Healthcare, Specialty Centers, Ambulatory Centers, and Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy) - Industry Trends and Forecast to 2030.
Asia-Pacific Antiviral Drugs Market Analysis and Insights
The increasing awareness about viral infections Asia-Pacific has enhanced the demand for the market. The rising healthcare expenditure for better health services also contributes to the market's growth. The major market players focus on various service launches and approvals during this crucial period. In addition, the increase in improved advancement of drug development techniques also contributes to the rising demand for antiviral drugs.
The Asia-Pacific antiviral drugs market is expected to grow in the forecast year due to the rise in market players and the availability of advanced services. Along with this, manufacturers are engaged in the developmental activity for launching novel services in the market. The increasing development in the field of drug development is further boosting the market growth. However, difficulties such as the lack of standardized protocols and the lack of skilled professionals might hamper the growth of the Asia-Pacific antiviral drugs market in the forecast period.
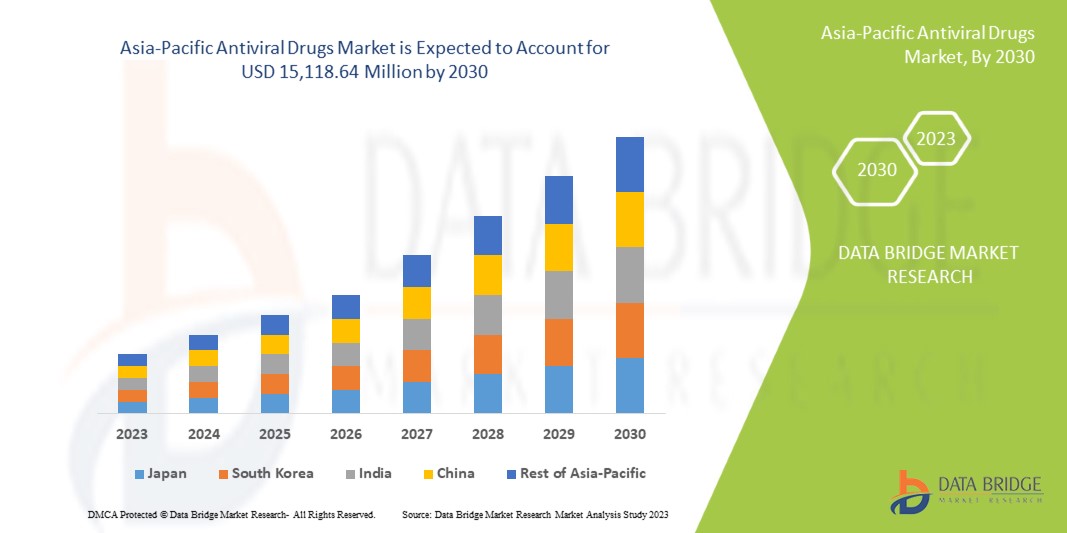
Increasing healthcare expenditure on advancement and drug development is expected to give opportunities to the market. However, the increasing use of alternative treatments may challenge market growth. Data Bridge Market Research analyzes that the Asia-Pacific antiviral drugs market is expected to reach a value of USD 15,118.64 million by 2030, with a CAGR of 6.7% during the forecast period.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
Indikation (Influenza, Humanes Immundefizienz-Virus (HIV), Hepatitis C-Virus (HCV), Respiratorisches Synzytial-Virus, Herpes-simplex-Virus, Humanes Cytomegalovirus (HCMV), Varizella-Zoster-Virus (VZV), Hepatitis B-Virus (HBV), Coronavirus-Infektion und andere), Patiententyp (Kinder, Erwachsene und geriatrische Patienten), Produkte (oral, topisch und parenteral), Arzneimitteltyp (Generika und Marken), Endverbraucher (Krankenhäuser, Kliniken, häusliche Pflege , Fachzentren, ambulante Zentren und andere), Vertriebskanal (Krankenhausapotheke, Online-Apotheke und Einzelhandelsapotheke) |
|
Abgedeckte Länder |
China, Japan, Indien, Südkorea, Australien, Singapur, Thailand, Malaysia, Indonesien, Philippinen, Vietnam und Rest des asiatisch-pazifischen Raums |
|
Abgedeckte Marktteilnehmer |
Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy's Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc. und Hetero. unter anderem |
Definition des Marktes für antivirale Medikamente im asiatisch-pazifischen Raum
Antivirale Medikamente sind Arzneimittel zur Behandlung von Virusinfektionen, indem sie die Replikation von Viren in Wirtszellen hemmen. Diese Medikamente zielen auf bestimmte Viren oder Virentypen ab und wirken, indem sie entweder das Eindringen des Virus in die Wirtszelle verhindern oder wichtige Enzyme oder Proteine blockieren, die für die Virusreplikation erforderlich sind. Im Gegensatz zu Antibiotika , die zur Behandlung bakterieller Infektionen eingesetzt werden, sind antivirale Medikamente im Allgemeinen weniger wirksam, da Viren eine viel einfachere Struktur haben und für ihre Replikation auf Wirtszellen angewiesen sind. Sie können jedoch dennoch bei der Behandlung einiger Virusinfektionen wie Grippe, Herpes und HIV nützlich sein.
Marktdynamik für antivirale Medikamente im asiatisch-pazifischen Raum
In diesem Abschnitt geht es um das Verständnis der Markttreiber, Chancen, Einschränkungen und Herausforderungen. All dies wird im Folgenden ausführlich erläutert:
Treiber
- Steigende Prävalenz von Virusinfektionen
Virusinfektionen haben in den letzten Jahrzehnten weltweit stetig zugenommen. Ein Virus, das in den Körper eindringt, dessen Zellen zur Replikation nutzt und sich ausbreitet, verursacht eine Virusinfektion. Virusinfektionen können verschiedene Symptome hervorrufen, von leichten bis hin zu schweren und in einigen Fällen sogar lebensbedrohlichen. Die Globalisierung ist eine der Hauptursachen für die Zunahme von Virusinfektionen. Menschen reisen und kommunizieren über Grenzen hinweg miteinander, wodurch die Welt vernetzter ist als je zuvor. Die Übertragung von Viren von einem Gebiet zum anderen hat sich aufgrund dieser verstärkten Vernetzung beschleunigt.
Daher ist die zunehmende Verbreitung von Virusinfektionen ein komplexes Problem mit vielen Faktoren. Globalisierung, Bevölkerungsdichte, Klimawandel und Antibiotikaresistenz beeinflussen die Verbreitung von Viren. Daher wird erwartet, dass dies das Marktwachstum ankurbelt.
- Fortschritte bei der Entwicklung neuer antiviraler Medikamente
Patienten mit Virusinfektionen werden antivirale Behandlungen verschrieben. Die Entwicklung neuartiger antiviraler Medikamente hat im Laufe der Zeit enorme Fortschritte gemacht. Diese Entwicklungen haben die Krankheitslast verringert, die Behandlung viraler Infektionen verbessert und Leben gerettet. Auf dem Gebiet neuer antiviraler Medikamente gibt es viel Entwicklung.
Fortschritte bei der Entwicklung neuer antiviraler Medikamente haben die Behandlung viraler Infektionen verbessert, die Krankheitslast verringert und dürften das Marktwachstum ankurbeln.
Zurückhaltung
- Hohe Kosten für antivirale Medikamente
Die hohen Kosten antiviraler Medikamente können erhebliche Folgen für Patienten und Gesundheitssysteme haben. Patienten, die sich diese Medikamente nicht leisten können, müssen möglicherweise auf eine Behandlung verzichten oder sind auf minderwertige Behandlungen angewiesen, was zu schlechteren Gesundheitsergebnissen führt. Darüber hinaus können die hohen Kosten antiviraler Medikamente die Gesundheitsbudgets belasten, insbesondere in Ländern mit begrenzten Ressourcen.
Daher ist zu erwarten, dass die hohen Kosten antiviraler Medikamente den Markt für antivirale Medikamente im asiatisch-pazifischen Raum bremsen werden.
Gelegenheit
-
Neue Wirkstoffverabreichungssysteme
Bei der Forschung zu antiviralen Medikamenten lag der Schwerpunkt auf der Entwicklung neuartiger Verabreichungsmechanismen. Im Vergleich zu herkömmlichen Verabreichungsmethoden bieten neuartige Verabreichungssysteme mehrere Vorteile, wie eine bessere Bioverfügbarkeit, eine maßgeschneiderte Verabreichung der Medikamente und weniger Nebenwirkungen.
Daher ist die Entwicklung neuartiger Arzneimittelverabreichungssysteme ein wichtiger Forschungsbereich für antivirale Medikamente. Arzneimittelverabreichungssysteme auf Nanopartikelbasis, Hydrogele, Dendrimere, Mikronadeln und zellpenetrierende Peptide sind einige vielversprechende Arzneimittelverabreichungssysteme, die für antivirale Medikamente untersucht wurden. Diese Verabreichungssysteme bieten mehrere Vorteile gegenüber herkömmlichen Arzneimittelverabreichungsmethoden und haben das Potenzial, die Wirksamkeit und Sicherheit antiviraler Medikamente zu verbessern, und dürften eine Chance für das Wachstum des Marktes schaffen.
Herausforderung
- Patentablauf bei antiviralen Medikamenten
Der Ablauf eines Patents führt dazu, dass der ursprüngliche Entwickler oder Patentinhaber sein ausschließliches Recht zur Herstellung und Vermarktung eines bestimmten Arzneimittels verliert. Der Ablauf eines Patents für antivirale Medikamente kann erhebliche Auswirkungen auf die Pharmabranche haben, da er möglicherweise Konkurrenz durch Hersteller von Generika nach sich zieht.
Die Patente für antivirale Medikamente erlöschen am Ende der Zeit, in der ihr Hersteller das alleinige Recht hat, das Medikament herzustellen und zu vermarkten. Nach Ablauf des Patents eines Medikaments können andere Hersteller Generika herstellen und vermarkten. Dies kann zu stärkerem Wettbewerb und niedrigeren Verbraucherpreisen führen. HIV, Hepatitis B und C, Herpes, Grippe und andere Viruserkrankungen werden alle mit antiviralen Medikamenten behandelt. Die Patente für antivirale Medikamente erlöschen je nach Medikament und Land zu unterschiedlichen Zeitpunkten. Medikamentenpatente werden normalerweise für 20 Jahre ab dem Anmeldedatum gewährt. Andere Hersteller können nach Ablauf des Patents Generika herstellen und vermarkten. Da der Hersteller nicht so viel für Marketing, Forschung und Entwicklung sowie klinische Studien ausgeben muss, sind Generika oft günstiger als Markenmedikamente.
Der Patentablauf bei antiviralen Medikamenten kann daher erhebliche Auswirkungen auf die Verfügbarkeit, Erschwinglichkeit und Zugänglichkeit dieser wichtigen Medikamente haben und dürfte eine Herausforderung für das Marktwachstum darstellen.
Jüngste Entwicklungen
- Im Januar 2023 gab Merck, bekannt als MSD, den erfolgreichen Abschluss des Barübernahmeangebots über eine Tochtergesellschaft für alle ausstehenden Stammaktien von Imago Biosciences, Inc. (Nasdaq: IMGO) zu einem Kaufpreis von 36,00 USD pro Aktie in bar, ohne Zinsen und vorbehaltlich des Abzugs etwaiger erforderlicher Quellensteuern bekannt. Die Übernahme wird zum Umsatzwachstum beitragen.
- Im April 2021 gab Zydus Pharmaceuticals, Inc. bekannt, dass es vom Drug Controller General of India (DCGI) eine eingeschränkte Notfallzulassung für das antivirale Medikament Virafin zur Behandlung mittelschwerer COVID-19-Infektionen erhalten habe. Dies wird dem Unternehmen helfen, seine Präsenz im asiatisch-pazifischen Raum und seinen Ruf in anderen Regionen der Welt zu stärken.
Marktumfang für antivirale Medikamente im asiatisch-pazifischen Raum
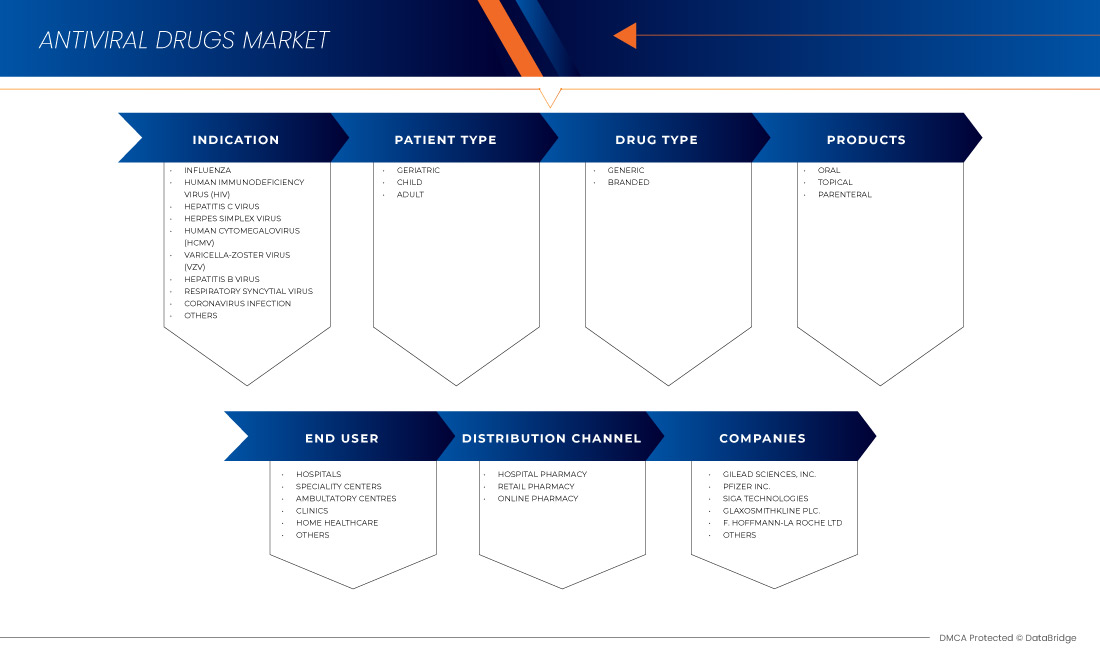
Der Markt für antivirale Medikamente im asiatisch-pazifischen Raum ist in sechs wichtige Segmente unterteilt, basierend auf Indikation, Patiententyp, Produkt, Medikamententyp, Endverbraucher und Vertriebskanal. Das Wachstum zwischen den Segmenten hilft Ihnen dabei, Nischenwachstumsbereiche und Strategien zur Marktbearbeitung zu analysieren und Ihre wichtigsten Anwendungsbereiche und die Unterschiede in Ihren Zielmärkten zu bestimmen.
Anzeige
- Grippe
- Menschliches Immundefizienz-Virus (HIV)
- Hepatitis C-Virus
- Herpes Simplex Virus
- Humanes Cytomegalievirus (HCMV)
- Varizella-Zoster-Virus (VZV)
- Hepatitis B Virus
- Respiratorisches Synzytial-Virus
- Coronavirus Infektion
- Sonstiges
Auf der Grundlage der Indikation ist der Markt für antivirale Medikamente im asiatisch-pazifischen Raum in Grippe, menschliches Immundefizienzvirus (HIV), Hepatitis C-Virus (HCV), Respiratorisches Synzytialvirus, Herpes-simplex-Virus, menschliches Cytomegalovirus (HCMV), Varizella-Zoster-Virus (VZV), Hepatitis B-Virus (HBV), Coronavirus-Infektion und andere unterteilt.
Patiententyp
- Kind
- Erwachsene
- Geriatrie
Auf der Grundlage des Patiententyps ist der Markt für antivirale Medikamente im asiatisch-pazifischen Raum in Kinder-, Erwachsenen- und geriatrische Medikamente segmentiert.
PRODUKTE
- Oral
- Aktuell
- Parenterale
Auf der Grundlage der Produkte ist der Markt für antivirale Medikamente im asiatisch-pazifischen Raum in orale, topische und parenterale Medikamente unterteilt.
Arzneimitteltyp
- Generisch
- Gebrandmarkt
Auf der Grundlage des Arzneimitteltyps ist der Markt für antivirale Medikamente im asiatisch-pazifischen Raum in Generika und Markenmedikamente segmentiert.
Endbenutzer
- Krankenhaus
- Kliniken
- Häusliche Gesundheitspflege
- Spezialzentren
- Ambulante Zentren
- Sonstiges
Auf der Grundlage des Endverbrauchers ist der Markt für antivirale Medikamente im asiatisch-pazifischen Raum in Krankenhäuser, Kliniken, häusliche Pflege, Fachzentren, ambulante Zentren und andere unterteilt.
Vertriebskanal
- Krankenhausapotheke
- Online-Apotheke
- Einzelhandelsapotheke
Auf der Grundlage der Vertriebskanäle ist der Markt für antivirale Medikamente im asiatisch-pazifischen Raum in Krankenhausapotheken, Online-Apotheken und Einzelhandelsapotheken unterteilt.
Regionale Analyse/Einblicke zum Markt für antivirale Medikamente im asiatisch-pazifischen Raum
Der Markt für antivirale Medikamente im asiatisch-pazifischen Raum ist basierend auf Indikation, Patiententyp, Produkt, Medikamententyp, Endverbraucher und Vertriebskanal in sechs wichtige Segmente unterteilt.
Die in diesem Marktbericht abgedeckten Länder sind China, Japan, Indien, Südkorea, Australien, Singapur, Thailand, Malaysia, Indonesien, die Philippinen, Vietnam und der restliche asiatisch-pazifische Raum.
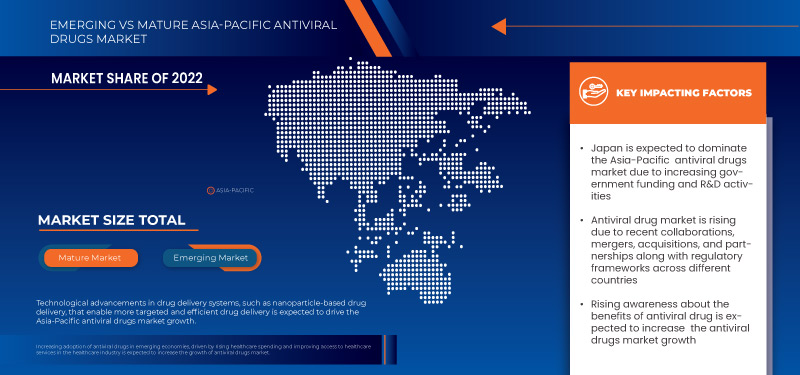
Im Jahr 2023 dominiert Japan den Asien-Pazifik-Raum aufgrund der starken Präsenz wichtiger Akteure und aufgrund der steigenden Nachfrage aus den Schwellenmärkten und der Expansion.
Der Länderabschnitt des Berichts enthält auch individuelle marktbeeinflussende Faktoren und Änderungen der Regulierung auf dem Inlandsmarkt, die sich auf die aktuellen und zukünftigen Markttrends auswirken. Datenpunkte wie Neuverkäufe, Ersatzverkäufe, Länderdemografie, Regulierungsgesetze und Import-/Exportzölle sind einige der wichtigsten Anhaltspunkte, die zur Prognose des Marktszenarios für einzelne Länder verwendet werden. Bei der Prognoseanalyse der Länderdaten werden auch die Präsenz und Verfügbarkeit von Marken aus dem asiatisch-pazifischen Raum und ihre Herausforderungen aufgrund großer oder geringer Konkurrenz durch lokale und inländische Marken sowie die Auswirkungen der Vertriebskanäle berücksichtigt.
Wettbewerbsumfeld und Marktanteilsanalyse für antivirale Medikamente im asiatisch-pazifischen Raum
Die Wettbewerbslandschaft des Marktes für antivirale Medikamente im asiatisch-pazifischen Raum bietet Einzelheiten nach Wettbewerbern. Die enthaltenen Einzelheiten umfassen Unternehmensübersicht, Unternehmensfinanzen, erzielten Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, Produktionsstandorte und -anlagen, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktzulassungen, Produktbreite und -breite, Anwendungsdominanz und Produkttyp-Lebenslinienkurve. Die oben angegebenen Datenpunkte beziehen sich nur auf den Fokus des Unternehmens auf den Markt für antivirale Medikamente im asiatisch-pazifischen Raum.
Some of the major players operating in the Asia-Pacific antiviral drugs market are Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy’s Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc, and Hetero. among others.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC ANTIVIRAL DRUGS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR ASIA-PACIFIC ANTIVIRAL DRUGS MARKET
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING PREVALENCE OF VIRAL INFECTIONS
8.1.2 ADVANCEMENTS IN NEW ANTIVIRAL DRUG DEVELOPMENT
8.1.3 GROWING DEMAND FOR COMBINATION THERAPIES
8.1.4 INCREASING GOVERNMENT FUNDING AND R&D ACTIVITIES
8.2 RESTRAINS
8.2.1 HIGH COST OF ANTIVIRAL DRUGS
8.2.2 EMERGENCE OF DRUG-RESISTANT STRAINS OF VIRUSES
8.3 OPPORTUNITIES
8.3.1 INCREASING COLLABORATION AND PARTNERSHIP AMONG KEY PLAYERS
8.3.2 RISING NOVEL DRUG DELIVERY SYSTEMS
8.3.3 DEVELOPMENT OF PERSONALIZED MEDICINES
8.4 CHALLENGES
8.4.1 PATENT EXPIRATION OF ANTIVIRAL DRUGS
8.4.2 DEMAND FOR ALTERNATIVE MEDICINES
9 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY INDICATION
9.1 OVERVIEW
9.2 INFLUENZA
9.2.1 NEURAMINIDASE INHIBITORS
9.2.1.1 OSELTAMIVIR
9.2.1.2 ZANAMIVIR
9.2.1.3 PERAMIVIR
9.2.1.4 LANINAMIVIR
9.2.2 M2 INHIBITORS
9.2.2.1 RIMANTADINE
9.2.2.2 OTHERS
9.2.3 RNA POLYMERASE INHIBITORS
9.2.3.1 FAVIPIRAVIR
9.2.3.2 BALOXAVIR MARBOXIL
9.3 HUMAN IMMUNODEFICIENCY VIRUS (HIV)
9.3.1 REVERSE TRANSCRIPTASE INHIBITORS
9.3.1.1 NUCLEOSIDE (NRTIS)
9.3.1.1.1 LAMIVUDINE
9.3.1.1.2 ABACAVIR
9.3.1.1.3 DIDANOSINE
9.3.1.1.4 OTHERS
9.3.1.2 NONNUCLEOSIDE (NNRTIS)
9.3.1.2.1 EFAVIRENZ
9.3.1.2.2 NEVIRAPINE
9.3.1.2.3 DELAVIRDINE
9.3.1.2.4 OTHERS
9.3.1.3 INTEGRASE
9.3.1.3.1 DOLUTEGRAVIR
9.3.1.3.2 ELVITEGRAVIR
9.3.1.3.3 RALTEGRAVIR
9.3.1.3.4 BICTEGRAVIR
9.3.1.4 NUCLEOTIDE
9.3.1.4.1 TENOFOVIR
9.3.1.4.2 OTHERS
9.3.1.5 INTERFERONS
9.3.1.5.1 ALPHA
9.3.1.5.2 BETA
9.3.1.5.3 GAMMA
9.3.1.6 GP41
9.3.1.6.1 ENFUVIRTIDE
9.3.1.6.2 OTHERS
9.3.2 PROTEASE
9.3.2.1 ATAZANAVIR
9.3.2.2 DARUNAVIR
9.3.2.3 LOPINAVIR
9.3.2.4 RITONAVIR
9.3.2.5 SAQUINAVIR
9.3.2.6 INDINAVIR
9.3.2.7 NELFINAVIR
9.3.2.8 TIPRANAVIR
9.3.2.9 AMPRENAVIR
9.4 HEPATITIS C VIRUS
9.4.1 NS5B POLYMERASE
9.4.1.1 SOFOSBUVIR
9.4.1.2 DASABUVIR
9.4.2 NS3/4A PROTEASE
9.4.2.1 DANOPREVIR
9.4.2.2 GLECAPREVIR
9.4.2.3 GRAZOPREVIR
9.4.2.4 PARITAPREVIR
9.4.2.5 SIMEPREVIR
9.4.3 NS5A PHOSPHOPROTEIN
9.4.3.1 LEDIPASVIR
9.4.3.2 VELPATASVIR
9.4.3.3 OMBITASVIR
9.4.3.4 ELBASVIR
9.4.3.5 DACLATASVIR
9.4.3.6 PIBRENTASVIR
9.4.4 NEURAMINIDASE
9.4.4.1 OSELTAMIVIR
9.4.4.2 ZANAMIVIR
9.4.4.3 PERAMIVIR
9.4.4.4 LANINAMIVIR
9.4.5 RNA POLYMERASE
9.4.5.1 BALOXAVIR MARBOXIL
9.4.5.2 FAVIPIRAVIR
9.4.6 MATRIX PROTEIN 2
9.4.6.1 RIMATIDINE
9.4.6.2 FAVIPIRAVIR
9.5 HERPES SIMPLEX VIRUS
9.5.1 DNA POLYMERASE UL30
9.5.1.1 ACICLOVIR
9.5.1.2 FAMCICLOVIR
9.5.1.3 VALACICLOVIR
9.5.1.4 PENCICLOVIR TRIFLURIDINE
9.5.1.5 BRIVUDINE
9.5.1.6 FOSCARNET
9.5.1.7 IDOXURIDINE
9.5.2 ENVELOPE PROTEINS
9.5.2.1 DOCOSANOL
9.5.2.2 OTHERS
9.6 HUMAN CYTOMEGALOVIRUS (HCMV)
9.6.1 GANCICLOVIR
9.6.2 VALGANCICLOVIR
9.6.3 CIDOFOVIR
9.6.4 FOSCARNET
9.6.5 FOMIVIRSEN
9.7 VARICELLA-ZOSTER VIRUS (VZV)
9.7.1 VALACICLOVIR
9.7.2 FAMCICLOVIR
9.7.3 ACICLOVIR
9.7.4 VIDARABINE
9.7.5 BRIVUDINE
9.8 HEPATITIS B VIRUS
9.8.1 ENTECAVIR
9.8.2 TENOFOVIR
9.8.3 TELBIVUDINE
9.8.4 TENOFOVIR ALAFENAMIDE
9.8.5 OTHERS
9.9 RESPIRATORY SYNCYTIAL VIRUS
9.9.1 RNA POLYMERASE
9.9.1.1 RIBAVIRIN
9.9.1.2 OTHERS
9.9.2 FUSION GLYCOPROTEIN
9.9.2.1 PALIVIZUMAB
9.9.2.2 OTHERS
9.1 CORONAVIRUS INFECTION
9.11 OTHERS
10 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 GERIATRIC
10.2.1 MALE
10.2.2 FEMALE
10.3 CHILD
10.3.1 MALE
10.3.2 FEMALE
10.4 ADULT
10.4.1 MALE
10.4.2 FEMALE
11 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY PRODUCTS
11.1 OVERVIEW
11.2 ORAL
11.2.1 SOLID
11.2.1.1 TABLETS
11.2.1.2 CAPSULES
11.2.1.3 OTHERS
11.2.2 SEMISOLID
11.2.2.1 GELS
11.2.2.2 EMULSIONS
11.2.2.3 ELIXIRS
11.2.2.4 OTHERS
11.2.3 LIQUID
11.2.3.1 SOLUTIONS
11.2.3.2 SYRUPS
11.2.3.3 OTHERS
11.3 TOPICAL
11.3.1 SEMI-SOLID
11.3.1.1 CREAM
11.3.1.2 OINTMENT
11.3.1.3 GELS
11.3.1.4 OTHERS
11.3.2 LIQUID
11.3.2.1 SOLUTIONS
11.3.2.2 SUSPENSIONS
11.3.3 SOLID
11.3.3.1 POWDERS
11.3.3.2 SUPPOSITORIES
11.3.3.3 ENEMA
11.3.3.4 OTHERS
11.4 PARENTERAL
11.4.1 CONVENTIONAL DRUG DELIVERY FORMUALTIONS
11.4.1.1 SOLUTIONS
11.4.1.2 RECONSTITUTED/LYOPHILIZED
11.4.1.3 SUSPENSIONS
11.4.1.4 EMULSIONS
11.4.1.5 OTHERS
11.4.2 NOVEL DRUG DELIVERY FORMULATIONS
11.4.3 COLLOIDAL DISPERSIONS
11.4.4 LONG ACTING INJECTION FORMULATION
12 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERIC
12.3 BRANDED
13 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITAL
13.3 SPECIALTY CENTERS
13.4 AMBULATORY CENTRES
13.5 CLINICS
13.6 HOME HEALTHCARE
13.7 OTHERS
14 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY REGION
15.1 ASIA-PACIFIC
15.1.1 JAPAN
15.1.2 CHINA
15.1.3 SOUTH KOREA
15.1.4 INDIA
15.1.5 AUSTRALIA
15.1.6 SINGAPORE
15.1.7 THAILAND
15.1.8 MALAYSIA
15.1.9 INDONESIA
15.1.10 PHILIPPINES
15.1.11 VIETNAM
15.1.12 REST OF ASIA-PACIFIC
16 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 GILEAD SCIENCES, INC. (2022)
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENT
18.2 PFIZER INC. (2022)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 SIGA TECHNOLOGIES (2022)
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 GLAXOSMITHKLINE PLC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENT
18.5 F. HOFFMANN-LA ROCHE LTD. (2022)
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBVIE INC.
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 AUROBINDO PHARMA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 AVET PHARMACEUTICALS INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BRISTOLL MYERS SQUIBB (2022)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BIOCRYST PHARMACEUTICALS, INC. (2022)
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 CIPLA INC. (2022)
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENT
18.12 DR. REDDY’S LABORATORIES LTD. (2022)
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 EMERGENT (2022)
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 HETERO.
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENT
18.15 JOHNSON & JOHNSON PRIVATE LIMITED (2022)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MACLEODS PHARMACEUTICALS LTD.
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MERCK & CO., INC, (2022)
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENT
18.18 MYLAN N.V (SUBSIDIARY OF VIATRIS) (2022)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 NAVINTA LLC.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENT
18.2 SUN PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 TEVA PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENT
18.22 ZYDUS PHARMACEUTICALS, INC. (2022)
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
Tabellenverzeichnis
TABLE 1 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 2 ASIA-PACIFIC INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 ASIA-PACIFIC INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 4 ASIA-PACIFIC NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 5 ASIA-PACIFIC M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 6 ASIA-PACIFIC M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 7 ASIA-PACIFIC HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 ASIA-PACIFIC HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 9 ASIA-PACIFIC REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 10 ASIA-PACIFIC NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 11 ASIA-PACIFIC NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 12 ASIA-PACIFIC INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 13 ASIA-PACIFIC NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 14 ASIA-PACIFIC INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 15 ASIA-PACIFIC GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 16 ASIA-PACIFIC PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 17 ASIA-PACIFIC HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 ASIA-PACIFIC HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 19 ASIA-PACIFIC NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 20 ASIA-PACIFIC NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 21 ASIA-PACIFIC NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 22 ASIA-PACIFIC NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 23 ASIA-PACIFIC RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 24 ASIA-PACIFIC MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 25 ASIA-PACIFIC HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 ASIA-PACIFIC HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 27 ASIA-PACIFIC DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 28 ASIA-PACIFIC ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 29 ASIA-PACIFIC HUMAN CYTOMEGALOVIRUS (HCMV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 ASIA-PACIFIC HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 31 ASIA-PACIFIC VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 ASIA-PACIFIC DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 33 ASIA-PACIFIC HEPATITIS B VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 ASIA-PACIFIC DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 35 ASIA-PACIFIC RESPIRATORY SYNCYTIAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 ASIA-PACIFIC RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 37 ASIA-PACIFIC RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 38 ASIA-PACIFIC FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 39 ASIA-PACIFIC CORONAVIRUS INFECTION IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 ASIA-PACIFIC OTHERS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021- 2030 (USD MILLION)
TABLE 42 ASIA-PACIFIC GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 ASIA-PACIFIC GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 44 ASIA-PACIFIC CHILD IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 ASIA-PACIFIC CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 46 ASIA-PACIFIC ADULT IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 ASIA-PACIFIC ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 48 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY PRODUCT, 2021- 2030 (USD MILLION)
TABLE 49 ASIA-PACIFIC ORAL IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 ASIA-PACIFIC ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 51 ASIA-PACIFIC SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 52 ASIA-PACIFIC SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 53 ASIA-PACIFIC LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 54 ASIA-PACIFIC TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 55 ASIA-PACIFIC TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 56 ASIA-PACIFIC SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 57 ASIA-PACIFIC LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 58 ASIA-PACIFIC SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 59 ASIA-PACIFIC PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 60 ASIA-PACIFIC PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 61 ASIA-PACIFIC CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 62 ASIA-PACIFIC NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 63 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021- 2030 (USD MILLION)
TABLE 64 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY END USER, 2021- 2030 (USD MILLION)
TABLE 65 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021- 2030 (USD MILLION)
TABLE 66 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 67 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 68 ASIA-PACIFIC INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 69 ASIA-PACIFIC NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 70 ASIA-PACIFIC M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 71 ASIA-PACIFIC RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 72 ASIA-PACIFIC HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 73 ASIA-PACIFIC REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 74 ASIA-PACIFIC NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 75 ASIA-PACIFIC NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 76 ASIA-PACIFIC INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 77 ASIA-PACIFIC NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 78 ASIA-PACIFIC INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 79 ASIA-PACIFIC GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 80 ASIA-PACIFIC PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 81 ASIA-PACIFIC HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 82 ASIA-PACIFIC NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 83 ASIA-PACIFIC NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 84 ASIA-PACIFIC NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 85 ASIA-PACIFIC NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 86 ASIA-PACIFIC RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 87 ASIA-PACIFIC MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 88 ASIA-PACIFIC HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 89 ASIA-PACIFIC DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 90 ASIA-PACIFIC ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 91 ASIA-PACIFIC HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 92 ASIA-PACIFIC VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 93 ASIA-PACIFIC DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 94 ASIA-PACIFIC RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 95 ASIA-PACIFIC RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 96 ASIA-PACIFIC FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 97 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 98 ASIA-PACIFIC GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 99 ASIA-PACIFIC CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 100 ASIA-PACIFIC ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 101 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 102 ASIA-PACIFIC ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 103 ASIA-PACIFIC SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 104 ASIA-PACIFIC SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 105 ASIA-PACIFIC LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 106 ASIA-PACIFIC TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 107 ASIA-PACIFIC SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 108 ASIA-PACIFIC LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 109 ASIA-PACIFIC SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 110 ASIA-PACIFIC PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 111 ASIA-PACIFIC CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 112 ASIA-PACIFIC NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 113 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 114 JAPAN ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 115 JAPAN INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 116 JAPAN NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 117 JAPAN M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 118 JAPAN RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 119 JAPAN HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 120 JAPAN REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 121 JAPAN NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 122 JAPAN NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 123 JAPAN INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 124 JAPAN NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 125 JAPAN INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 126 JAPAN GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 127 JAPAN PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 128 JAPAN HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 129 JAPAN NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 130 JAPAN NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 131 JAPAN NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 132 JAPAN NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 133 JAPAN RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 134 JAPAN MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 135 JAPAN MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 136 JAPAN MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 137 JAPAN MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 138 JAPAN MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 139 JAPAN ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 140 JAPAN HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 141 JAPAN VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 142 JAPAN DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 143 JAPAN RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 144 JAPAN RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 145 JAPAN FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 146 JAPAN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 147 JAPAN GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 148 JAPAN CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 149 JAPAN ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 150 JAPAN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 151 JAPAN ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 152 JAPAN SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 153 JAPAN SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 154 JAPAN LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 155 JAPAN TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 156 JAPAN SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 157 JAPAN LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 158 JAPAN SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 159 JAPAN PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 160 JAPAN CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 161 JAPAN NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 162 JAPAN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 163 JAPAN ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 164 JAPAN ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 165 CHINA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 166 CHINA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 167 CHINA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 168 CHINA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 169 CHINA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 170 CHINA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 171 CHINA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 172 CHINA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 173 CHINA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 174 CHINA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 175 CHINA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 176 CHINA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 177 CHINA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 178 CHINA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 179 CHINA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 180 CHINA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 181 CHINA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 182 CHINA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 183 CHINA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 184 CHINA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 185 CHINA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 186 CHINA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 187 CHINA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 188 CHINA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 189 CHINA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 190 CHINA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 191 CHINA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 192 CHINA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 193 CHINA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 194 CHINA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 195 CHINA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 196 CHINA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 197 CHINA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 198 CHINA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 199 CHINA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 200 CHINA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 201 CHINA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 202 CHINA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 203 CHINA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 204 CHINA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 205 CHINA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 206 CHINA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 207 CHINA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 208 CHINA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 209 CHINA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 210 CHINA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 211 CHINA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 212 CHINA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 CHINA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 214 SOUTH KOREA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 215 SOUTH KOREA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 216 SOUTH KOREA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 217 SOUTH KOREA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 218 SOUTH KOREA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 219 SOUTH KOREA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 220 SOUTH KOREA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 221 SOUTH KOREA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 222 SOUTH KOREA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 223 SOUTH KOREA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 224 SOUTH KOREA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 225 SOUTH KOREA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 226 SOUTH KOREA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 227 SOUTH KOREA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 228 SOUTH KOREA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 229 SOUTH KOREA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 230 SOUTH KOREA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 231 SOUTH KOREA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 232 SOUTH KOREA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 233 SOUTH KOREA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 234 SOUTH KOREA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 235 SOUTH KOREA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 236 SOUTH KOREA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 237 SOUTH KOREA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 238 SOUTH KOREA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 239 SOUTH KOREA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 240 SOUTH KOREA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 241 SOUTH KOREA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 242 SOUTH KOREA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 243 SOUTH KOREA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 244 SOUTH KOREA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 245 SOUTH KOREA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 246 SOUTH KOREA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 247 SOUTH KOREA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 248 SOUTH KOREA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 249 SOUTH KOREA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 250 SOUTH KOREA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 251 SOUTH KOREA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 252 SOUTH KOREA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 253 SOUTH KOREA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 254 SOUTH KOREA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 255 SOUTH KOREA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 256 SOUTH KOREA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 257 SOUTH KOREA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 258 SOUTH KOREA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 259 SOUTH KOREA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 260 SOUTH KOREA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 261 SOUTH KOREA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 262 SOUTH KOREA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 263 INDIA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 264 INDIA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 265 INDIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 266 INDIA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 267 INDIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 268 INDIA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 269 INDIA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 270 INDIA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 271 INDIA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 272 INDIA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 273 INDIA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 274 INDIA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 275 INDIA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 276 INDIA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 277 INDIA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 278 INDIA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 279 INDIA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 280 INDIA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 281 INDIA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 282 INDIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 283 INDIA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 284 INDIA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 285 INDIA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 286 INDIA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 287 INDIA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 288 INDIA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 289 INDIA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 290 INDIA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 291 INDIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 292 INDIA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 293 INDIA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 294 INDIA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 295 INDIA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 296 INDIA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 297 INDIA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 298 INDIA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 299 INDIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 300 INDIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 301 INDIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 302 INDIA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 303 INDIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 304 INDIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 305 INDIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 306 INDIA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 307 INDIA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 308 INDIA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 309 INDIA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 310 INDIA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 311 INDIA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 312 AUSTRALIA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 313 AUSTRALIA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 314 AUSTRALIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 315 AUSTRALIA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 316 AUSTRALIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 317 AUSTRALIA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 318 AUSTRALIA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 319 AUSTRALIA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 320 AUSTRALIA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 321 AUSTRALIA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 322 AUSTRALIA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 323 AUSTRALIA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 324 AUSTRALIA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 325 AUSTRALIA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 326 AUSTRALIA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 327 AUSTRALIA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 328 AUSTRALIA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 329 AUSTRALIA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 330 AUSTRALIA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 331 AUSTRALIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 332 AUSTRALIA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 333 AUSTRALIA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 334 AUSTRALIA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 335 AUSTRALIA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 336 AUSTRALIA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 337 AUSTRALIA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 338 AUSTRALIA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 339 AUSTRALIA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 340 AUSTRALIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 341 AUSTRALIA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 342 AUSTRALIA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 343 AUSTRALIA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 344 AUSTRALIA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 345 AUSTRALIA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 346 AUSTRALIA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 347 AUSTRALIA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 348 AUSTRALIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 349 AUSTRALIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 350 AUSTRALIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 351 AUSTRALIA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 352 AUSTRALIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 353 AUSTRALIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 354 AUSTRALIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 355 AUSTRALIA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 356 AUSTRALIA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 357 AUSTRALIA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 358 AUSTRALIA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 359 AUSTRALIA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 360 AUSTRALIA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 361 SINGAPORE ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 362 SINGAPORE INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 363 SINGAPORE NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 364 SINGAPORE M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 365 SINGAPORE RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 366 SINGAPORE HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 367 SINGAPORE REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 368 SINGAPORE NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 369 SINGAPORE NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 370 SINGAPORE INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 371 SINGAPORE NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 372 SINGAPORE INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 373 SINGAPORE GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 374 SINGAPORE PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 375 SINGAPORE HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 376 SINGAPORE NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 377 SINGAPORE NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 378 SINGAPORE NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 379 SINGAPORE NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 380 SINGAPORE RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 381 SINGAPORE MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 382 SINGAPORE HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 383 SINGAPORE DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 384 SINGAPORE ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 385 SINGAPORE HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 386 SINGAPORE VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 387 SINGAPORE DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 388 SINGAPORE RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 389 SINGAPORE RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 390 SINGAPORE FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 391 SINGAPORE ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 392 SINGAPORE GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 393 SINGAPORE CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 394 SINGAPORE ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 395 SINGAPORE ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 396 SINGAPORE ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 397 SINGAPORE SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 398 SINGAPORE SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 399 SINGAPORE LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 400 SINGAPORE TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 401 SINGAPORE SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 402 SINGAPORE LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 403 SINGAPORE SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 404 SINGAPORE PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 405 SINGAPORE CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 406 SINGAPORE NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 407 SINGAPORE ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 408 SINGAPORE ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 409 SINGAPORE ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 410 THAILAND ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 411 THAILAND INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 412 THAILAND NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 413 THAILAND M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 414 THAILAND RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 415 THAILAND HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 416 THAILAND REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 417 THAILAND NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 418 THAILAND NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 419 THAILAND INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 420 THAILAND NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 421 THAILAND INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 422 THAILAND GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 423 THAILAND PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 424 THAILAND HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 425 THAILAND NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 426 THAILAND NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 427 THAILAND NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 428 THAILAND NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 429 THAILAND RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 430 THAILAND MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 431 THAILAND HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 432 THAILAND DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 433 THAILAND ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 434 THAILAND HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 435 THAILAND VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 436 THAILAND DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 437 THAILAND RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 438 THAILAND RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 439 THAILAND FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 440 THAILAND ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 441 THAILAND GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 442 THAILAND CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 443 THAILAND ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 444 THAILAND ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 445 THAILAND ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 446 THAILAND SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 447 THAILAND SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 448 THAILAND LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 449 THAILAND TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 450 THAILAND SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 451 THAILAND LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 452 THAILAND SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 453 THAILAND PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 454 THAILAND CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 455 THAILAND NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 456 THAILAND ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 457 THAILAND ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 458 THAILAND ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 459 MALAYSIA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 460 MALAYSIA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 461 MALAYSIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 462 MALAYSIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 463 MALAYSIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 464 MALAYSIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 465 MALAYSIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 466 MALAYSIA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 467 MALAYSIA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 468 MALAYSIA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 469 MALAYSIA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 470 MALAYSIA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 471 MALAYSIA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 472 MALAYSIA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 473 MALAYSIA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 474 MALAYSIA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 475 MALAYSIA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 476 MALAYSIA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 477 MALAYSIA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 478 MALAYSIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 479 MALAYSIA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 480 MALAYSIA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 481 MALAYSIA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 482 MALAYSIA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 483 MALAYSIA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 484 MALAYSIA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 485 MALAYSIA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 486 MALAYSIA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 487 MALAYSIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 488 MALAYSIA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 489 MALAYSIA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 490 MALAYSIA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 491 MALAYSIA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 492 MALAYSIA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 493 MALAYSIA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 494 MALAYSIA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 495 MALAYSIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 496 MALAYSIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 497 MALAYSIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 498 MALAYSIA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 499 MALAYSIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 500 MALAYSIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 501 MALAYSIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 502 MALAYSIA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 503 MALAYSIA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 504 MALAYSIA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 505 MALAYSIA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 506 MALAYSIA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 507 MALAYSIA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 508 INDONESIA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 509 INDONESIA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 510 INDONESIA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 511 INDONESIA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 512 INDONESIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 513 INDONESIA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 514 INDONESIA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 515 INDONESIA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 516 INDONESIA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 517 INDONESIA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 518 INDONESIA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 519 INDONESIA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 520 INDONESIA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 521 INDONESIA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 522 INDONESIA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 523 INDONESIA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 524 INDONESIA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 525 INDONESIA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 526 INDONESIA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 527 INDONESIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 528 INDONESIA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 529 INDONESIA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 530 INDONESIA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 531 INDONESIA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 532 INDONESIA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 533 INDONESIA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 534 INDONESIA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 535 INDONESIA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 536 INDONESIA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 537 INDONESIA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 538 INDONESIA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 539 INDONESIA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 540 INDONESIA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 541 INDONESIA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 542 INDONESIA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 543 INDONESIA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 544 INDONESIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 545 INDONESIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 546 INDONESIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 547 INDONESIA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 548 INDONESIA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 549 INDONESIA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 550 INDONESIA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 551 INDONESIA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 552 INDONESIA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 553 INDONESIA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 554 INDONESIA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 555 INDONESIA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 556 INDONESIA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 557 PHILIPPINES ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 558 PHILIPPINES INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 559 PHILIPPINES NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 560 PHILIPPINES M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 561 PHILIPPINES RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 562 PHILIPPINES HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 563 PHILIPPINES REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 564 PHILIPPINES NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 565 PHILIPPINES INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 566 PHILIPPINES NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 567 PHILIPPINES INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 568 PHILIPPINES GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 569 PHILIPPINES PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 570 PHILIPPINES HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 571 PHILIPPINES NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 572 PHILIPPINES NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 573 PHILIPPINES NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 574 PHILIPPINES NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 575 PHILIPPINES RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 576 PHILIPPINES MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 577 PHILIPPINES HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 578 PHILIPPINES DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 579 PHILIPPINES ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 580 PHILIPPINES HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 581 PHILIPPINES VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 582 PHILIPPINES DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 583 PHILIPPINES RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 584 PHILIPPINES RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 585 PHILIPPINES FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 586 PHILIPPINES ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 587 PHILIPPINES GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 588 PHILIPPINES CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 589 PHILIPPINES ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 590 PHILIPPINES ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 591 PHILIPPINES ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 592 PHILIPPINES SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 593 PHILIPPINES SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 594 PHILIPPINES LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 595 PHILIPPINES TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 596 PHILIPPINES SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 597 PHILIPPINES LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 598 PHILIPPINES SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 599 PHILIPPINES PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 600 PHILIPPINES CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 601 PHILIPPINES NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 602 PHILIPPINES ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 603 PHILIPPINES ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 604 PHILIPPINES ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 605 VIETNAM ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 606 VIETNAM INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 607 VIETNAM NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 608 VIETNAM M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 609 VIETNAM RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 610 VIETNAM HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 611 VIETNAM REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 612 VIETNAM NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 613 VIETNAM NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 614 VIETNAM INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 615 VIETNAM NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 616 VIETNAM INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 617 VIETNAM GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 618 VIETNAM PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 619 VIETNAM HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 620 VIETNAM NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 621 VIETNAM NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 622 VIETNAM NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 623 VIETNAM NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 624 VIETNAM RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 625 VIETNAM MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 626 VIETNAM HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 627 VIETNAM DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 628 VIETNAM ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 629 VIETNAM HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 630 VIETNAM VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 631 VIETNAM DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 632 VIETNAM RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 633 VIETNAM RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 634 VIETNAM FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 635 VIETNAM ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 636 VIETNAM GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 637 VIETNAM CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 638 VIETNAM ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 639 VIETNAM ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 640 VIETNAM ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 641 VIETNAM SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 642 VIETNAM SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 643 VIETNAM LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 644 VIETNAM TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 645 VIETNAM SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 646 VIETNAM LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 647 VIETNAM SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 648 VIETNAM PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 649 VIETNAM CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 650 VIETNAM NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 651 VIETNAM ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 652 VIETNAM ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 653 VIETNAM ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 654 REST OF ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
Abbildungsverzeichnis
FIGURE 1 ASIA-PACIFIC ANTIVIRAL DRUGS: SEGMENTATION
FIGURE 2 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: ASIA-PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE OF VIRAL INFECTIONS IS EXPECTED TO DRIVE THE ASIA-PACIFIC ANTIVIRAL DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INFLUENZA SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC ANTIVIRAL DRUGS MARKET IN 2023 & 2030
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE ASIA-PACIFIC ANTIVIRAL DRUGS MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 14 ASIA-PACIFIC IS THE FASTEST-GROWING MARKET FOR ASIA-PACIFIC ANTIVIRAL DRUGS MARKET MANUFACTURERS IN THE FORECAST PERIOD
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE ASIA-PACIFIC ANTIVIRAL DRUGS MARKET
FIGURE 16 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY INDICATION , 2022
FIGURE 17 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY INDICATION , 2023-2030 (USD MILLION)
FIGURE 18 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY INDICATION , CAGR (2023-2030)
FIGURE 19 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY INDICATION , LIFELINE CURVE
FIGURE 20 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2022
FIGURE 21 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2023-2030 (USD MILLION)
FIGURE 22 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, CAGR (2023-2030)
FIGURE 23 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2022
FIGURE 25 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 26 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 27 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2022
FIGURE 29 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2023-2030 (USD MILLION)
FIGURE 30 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, CAGR (2023-2030)
FIGURE 31 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY END USER, 2022
FIGURE 33 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 34 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 35 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY END USER, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 37 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 38 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 39 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 40 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: SNAPSHOT (2022)
FIGURE 41 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022)
FIGURE 42 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 43 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 44 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: INDICATION (2023-2030)
FIGURE 45 ASIA-PACIFIC ANTIVIRAL DRUGS MARKET: COMPANY SHARE 2022 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.