Die steigende Zahl von Schlaganfällen ist ein wichtiger Treiber des globalen Schlaganfallmarktes und beeinflusst sowohl die Nachfrage nach Behandlung als auch nach Gesundheitsinfrastruktur. Schlaganfälle, eine der häufigsten Ursachen für Behinderungen und Todesfälle weltweit, treten aufgrund verschiedener Risikofaktoren wie alternder Bevölkerung, Bewegungsmangel, Bluthochdruck, Diabetes, Rauchen und ungesunder Ernährung immer häufiger auf. Mit steigender Lebenserwartung und alternder Bevölkerung steigt auch die Prävalenz von Erkrankungen, die zu Schlaganfällen beitragen, wie Bluthochdruck und Vorhofflimmern. Dies führt dazu, dass mehr Menschen einen Schlaganfall erleiden und sofortige medizinische Versorgung und langfristige Rehabilitation benötigen.
Da die weltweite Belastung durch Schlaganfälle weiter zunimmt, konzentrieren sich Regierungen, Gesundheitsorganisationen und Pharmaunternehmen auf die Verbesserung der Schlaganfallprävention, Frühdiagnose und Behandlung. Dieser Fokus hat zu Innovationen in der Schlaganfalltherapie geführt und den Markt für schlaganfallbezogene Gesundheitsprodukte und -dienstleistungen erweitert. Darüber hinaus tragen Aufklärungskampagnen und Gesundheitsinitiativen zur Früherkennung und Behandlung bei und sorgen dafür, dass mehr Patienten Schlaganfälle mit verbesserter Lebensqualität überleben. Insgesamt ist die steigende Zahl von Schlaganfällen ein entscheidender Treiber des Marktwachstums und schafft Nachfrage nach innovativen Behandlungen, Technologien und Rehabilitationslösungen.
Vollständigen Bericht abrufen unter https://www.databridgemarketresearch.com/reports/global-stroke-market
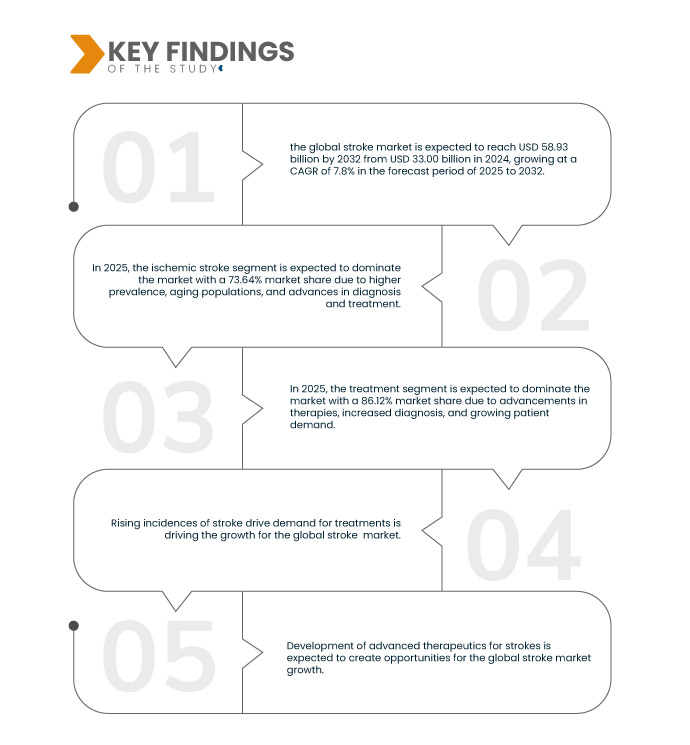
Data Bridge Market Research analysiert, dass der globale Schlaganfallmarkt von 33,00 Milliarden US-Dollar im Jahr 2024 auf 58,93 Milliarden US-Dollar im Jahr 2032 anwachsen dürfte, was einem durchschnittlichen jährlichen Wachstumswachstum von 7,8 % im Prognosezeitraum von 2025 bis 2032 entspricht.
Wichtigste Ergebnisse der Studie
Steigende Zahl von Patienten mit Bluthochdruck und koronarer Herzkrankheit
Hypertonie, allgemein als Bluthochdruck bekannt, ist eine Erkrankung, die durch einen erhöhten Druck des Blutes auf die Arterienwände gekennzeichnet ist. Sie wird typischerweise durch einen Blutdruckwert von 130/80 mmHg oder höher definiert und kann, je nach zugrunde liegender Ursache, als essentiell (primär) oder sekundär eingestuft werden. Dauerhafter Bluthochdruck kann zu einer Reihe von ernstzunehmenden Gesundheitsproblemen führen, von denen die koronare Herzkrankheit zu den schwerwiegendsten gehört. Die koronare Herzkrankheit, auch als koronare Arterienerkrankung bekannt, entsteht durch die allmähliche Ansammlung von Fettablagerungen (Arteriosklerose) in den Koronararterien, die den Herzmuskel mit Sauerstoff und Nährstoffen versorgen. Wenn sich diese Arterien verengen oder verstopfen, verringert sich der Blutfluss zum Herzen, was zu Brustschmerzen (Angina pectoris) und in schweren Fällen zu Herzinfarkten führt.
Schlaganfälle stehen aufgrund gemeinsamer Risikofaktoren und zugrunde liegender physiologischer Mechanismen in engem Zusammenhang mit der zunehmenden Zahl von Patienten mit Bluthochdruck und koronarer Herzkrankheit (KHK). Hypertonie oder Bluthochdruck erhöht das Schlaganfallrisiko erheblich, da er die Blutgefäße schädigt und die Wahrscheinlichkeit von Blutgerinnseln erhöht. Länger anhaltender Bluthochdruck kann zu Arteriosklerose führen, der Ansammlung von Fettablagerungen in den Arterien, einschließlich derjenigen, die das Gehirn versorgen. Dies kann zu verengten Blutgefäßen und einer verminderten Durchblutung des Gehirns führen und so das Risiko sowohl ischämischer (durch Verstopfungen verursachter) als auch hämorrhagischer (durch Blutungen verursachter) Schlaganfälle erhöhen. Hoher Blutdruck kann zudem die Blutgefäße im Gehirn schädigen und sie anfälliger für Ruptur oder Verstopfung machen, was das Schlaganfallrisiko weiter erhöht.
Berichtsumfang und Marktsegmentierung
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2025 bis 2032
|
Basisjahr
|
2024
|
Historisches Jahr
|
2023 (Anpassbar 2013–2017)
|
Quantitative Einheiten
|
Umsatz in Milliarden USD
|
Abgedeckte Segmente
|
Typ (ischämischer Schlaganfall, vorübergehende ischämische Attacke (TIA) und hämorrhagischer Schlaganfall ), Diagnose und Behandlung (Behandlung und Diagnose), Geschlecht (weiblich und männlich), Endbenutzer (Krankenhäuser und Kliniken, Fachkliniken, ambulante chirurgische Zentren, häusliche Pflege, Labore und andere), Vertriebskanal (direkt, Einzelhandel und online)
|
Abgedeckte Länder
|
USA, Kanada, Mexiko, Deutschland, Frankreich, Großbritannien, Italien, Spanien, Russland, Niederlande, Schweiz, Türkei, Österreich, Irland, Norwegen, Polen, übriges Europa, Japan, China, Indien, Südkorea, Australien, Singapur, Thailand, Malaysia, Indonesien, Vietnam, Philippinen, Taiwan, übriger Asien-Pazifik-Raum, Brasilien, Argentinien, Chile, Peru, übriges Südamerika, Südafrika, Saudi-Arabien, Vereinigte Arabische Emirate, Ägypten, Kuwait, Israel und übriger Naher Osten und Afrika
|
Abgedeckte Marktteilnehmer
|
Bristol-Myers Squibb Company (USA), Boehringer Ingelheim International GmbH (Deutschland), F. Hoffmann-La Roche Ltd (Schweiz), DAIICHI SANKYO COMPANY, LIMITED (Japan), Sanofi (Frankreich), Johnson & Johnson Services, Inc. (USA), Bayer AG (Deutschland), Sandoz AG (Schweiz), Pfizer Inc. (USA), Medtronic (Irland), Abbott (USA), Viatris Inc. (USA), AstraZeneca (Großbritannien), Penumbra, Inc. (USA), GLENMARK PHARMACEUTICALS LTD (Indien), Fresenius SE & Co. KGaA (Deutschland), Teva Pharmaceuticals USA, Inc. (Israel), Lupin (Indien) und Amneal Pharmaceuticals LLC (USA) unter anderem
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
|
Segmentanalyse
Der globale Schlaganfallmarkt ist basierend auf Typ, Diagnose und Behandlung, Geschlecht, Endbenutzer und Vertriebskanal in fünf wichtige Segmente unterteilt.
- Der globale Schlaganfallmarkt ist nach Art in ischämischen Schlaganfall, hämorrhagischen Schlaganfall und vorübergehenden ischämischen Schlaganfall (TIA) unterteilt.
Im Jahr 2025 wird das Segment ischämischer Schlaganfall voraussichtlich den globalen Schlaganfallmarkt dominieren
Im Jahr 2025 wird das Segment der ischämischen Schlaganfälle voraussichtlich mit einem Marktanteil von 73,64 % den Markt dominieren, was auf die höhere Prävalenz, die alternde Bevölkerung und die Fortschritte bei Diagnose und Behandlung zurückzuführen ist.
- Auf der Grundlage von Diagnose und Behandlung ist der Markt in Behandlung und Diagnose segmentiert
Im Jahr 2025 wird das Behandlungssegment voraussichtlich den globalen Schlaganfallmarkt dominieren
Im Jahr 2025 wird das Behandlungssegment voraussichtlich mit einem Marktanteil von 86,12 % den Markt dominieren, was auf Fortschritte bei den Therapien, eine verbesserte Diagnose und eine steigende Patientennachfrage zurückzuführen ist.
- Der globale Schlaganfallmarkt ist nach Geschlecht in Frauen und Männer segmentiert. Im Jahr 2025 wird das weibliche Segment voraussichtlich mit einem Marktanteil von 56,84 % den Markt dominieren.
- Der globale Schlaganfallmarkt ist nach Endverbrauchern segmentiert in Krankenhäuser und Kliniken, Fachkliniken, ambulante chirurgische Zentren, häusliche Pflege, Labore und weitere. Im Jahr 2025 wird das Segment Krankenhäuser und Kliniken voraussichtlich mit einem Marktanteil von 51,57 % den Markt dominieren.
- Der globale Schlaganfallmarkt ist nach Vertriebskanälen in Direktvertrieb, Einzelhandel und Online-Vertrieb unterteilt. Im Jahr 2025 wird das Direktsegment voraussichtlich mit einem Marktanteil von 79,35 % den Markt dominieren.
Hauptakteure
Data Bridge Market Research analysiert Bristol-Myers Squibb Company (USA), Boehringer Ingelheim International GmbH (Deutschland), F. Hoffmann-La Roche Ltd (Schweiz), DAIICHI SANKYO COMPANY, LIMITED (Japan) und Sanofi (Frankreich) als die wichtigsten Marktteilnehmer.
Marktentwicklungen
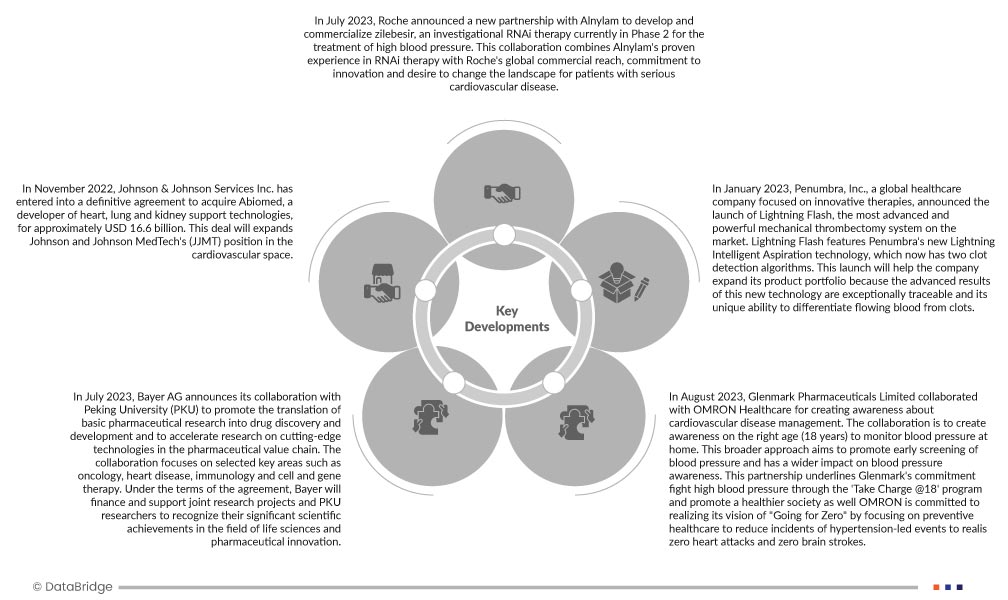
- Im Juli 2023 gab Roche eine neue Partnerschaft mit Alnylam zur Entwicklung und Vermarktung von Zilebesir bekannt, einer RNAi-Therapie zur Behandlung von Bluthochdruck, die sich derzeit in Phase 2 befindet. Diese Zusammenarbeit verbindet Alnylams langjährige Erfahrung in der RNAi-Therapie mit Roches globaler Marktpräsenz, seinem Engagement für Innovation und seinem Anspruch, die Situation für Patienten mit schweren Herz-Kreislauf-Erkrankungen zu verändern.
- Im September 2020 gab Daiichi Sankyo Company Limited bekannt, dass sie in Japan einen ergänzenden Antrag auf eine erweiterte Zulassung des Antikoagulans Edoxaban (Edoxabanbenzoathydrat) bei älteren Patienten mit nicht-valvulärer Insuffizienz und starkem Blutungsrisiko eingereicht hat. Dieser Antrag basiert auf den Ergebnissen einer japanischen klinischen Phase-3-Studie (ELDERCARE-AF-Studie) an 984 Patienten mit nicht-valvulärem Vorhofflimmern, die mindestens 80 Jahre alt sind, ein hohes Blutungsrisiko aufweisen und für die andere verfügbare Antikoagulanzientherapien nicht geeignet sind. Daiichi Sankyo möchte mit einer neuen Behandlungsoption zur Behandlung älterer Patienten mit nicht-valvulärem Vorhofflimmern beitragen.
- Im Mai 2021 gab Abbott die CE-Kennzeichnung für sein neuestes System zur transkatheteralen Aortenklappenimplantation (TAVI), Navitor, bekannt. Damit ist das minimalinvasive Gerät nun auch für Menschen mit schwerer Aortenstenose und hohem oder extremem Operationsrisiko in Europa verfügbar. Mit der Navitor-Klappe treibt das Unternehmen TAVI-Therapien (auch TAVR oder transkatheteraler Aortenklappenersatz genannt) mit Innovationen voran, darunter ein einzigartiges Design zur Verhinderung von Blutleckagen um die Klappe herum. Diese Zulassung stärkt das Portfolio des Unternehmens an minimalinvasiven Angeboten im Bereich der strukturellen Herztherapie und bietet neue Optionen und Verbesserungen zur Behandlung einer lebensbedrohlichen Herzerkrankung.
- Im April 2021 gab Abbott die CE-Kennzeichnung für sein TriClip Transkatheter-Trikuspidalklappen-Reparatursystem der nächsten Generation bekannt. Es ist das erste minimalinvasive Trikuspidalklappen-Reparatursystem seiner Art in Europa zur Behandlung einer Trikuspidalklappeninsuffizienz (TR). Die Clip-basierte Therapie, bekannt als TriClip G4, ist eine nicht-chirurgische Herzklappenreparaturoption, die speziell für die Behandlung einer TR (einer undichten Trikuspidalklappe) entwickelt wurde. Sie ermöglicht es Ärzten, die Reparatur der Klappe individuell an die individuelle Anatomie jedes Patienten anzupassen. Diese Innovation steigert die Leistungsfähigkeit des Unternehmens und verbessert die Fähigkeit von Kardiologen, die Trikuspidalklappe sicher und effektiv zu reparieren.
- Im Juli 2023 gibt die Bayer AG ihre Zusammenarbeit mit der Peking University (PKU) bekannt, um die Translation pharmazeutischer Grundlagenforschung in die Arzneimittelforschung und -entwicklung zu fördern und die Forschung an Spitzentechnologien in der pharmazeutischen Wertschöpfungskette zu beschleunigen. Die Zusammenarbeit konzentriert sich auf ausgewählte Schlüsselbereiche wie Onkologie, Herzerkrankungen, Immunologie sowie Zell- und Gentherapie. Im Rahmen der Vereinbarung wird Bayer gemeinsame Forschungsprojekte und PKU-Forscher finanzieren und unterstützen, um ihre bedeutenden wissenschaftlichen Leistungen in den Bereichen Biowissenschaften und pharmazeutische Innovation zu würdigen. Die Vereinbarung basiert auf einer strategischen akademischen Partnerschaft zwischen Bayer und der PKU, die ein gemeinsames Forschungszentrum an der Peking University eingerichtet hat, um die Translation von Arzneimittelentwicklung und -forschung in Medikamente für Patienten voranzutreiben.
Regionale Analyse
Geografisch betrachtet sind dies die folgenden Länder, die im globalen Schlaganfallmarktbericht abgedeckt sind: die USA, Kanada, Mexiko, Deutschland, Frankreich, Großbritannien, Italien, Spanien, Russland, die Niederlande, die Schweiz, die Türkei, Österreich, Irland, Norwegen, Polen, das übrige Europa, Japan, China, Indien, Südkorea, Australien, Singapur, Thailand, Malaysia, Indonesien, Vietnam, die Philippinen, Taiwan, der übrige asiatisch-pazifische Raum, Brasilien, Argentinien, Chile, Peru, das übrige Südamerika, Südafrika, Saudi-Arabien, die Vereinigten Arabischen Emirate, Ägypten, Kuwait, Israel sowie der übrige Nahe Osten und Afrika.
Laut Marktforschungsanalyse von Data Bridge:
Nordamerika wird voraussichtlich den globalen Schlaganfallmarkt dominieren
Nordamerika dominiert den globalen Schlaganfallmarkt, was auf die fortschrittlichen Gesundheitssysteme, die hohe Schlaganfallrate und die hohe Akzeptanz der Behandlungsmethoden zurückzuführen ist.
Asien-Pazifik ist die am schnellsten wachsende Region im globalen Schlaganfallmarkt
Der asiatisch-pazifische Raum ist die am schnellsten wachsende Region auf dem globalen Schlaganfallmarkt. Gründe hierfür sind die alternde Bevölkerung, steigende Risikofaktoren und eine Verbesserung der Gesundheitsinfrastruktur.
Für detailliertere Informationen zum globalen Schlaganfallmarkt klicken Sie hier – https://www.databridgemarketresearch.com/reports/global-stroke-market