Die zunehmende Verbreitung von Hepatitis-B -Infektionen ist ein wichtiger Treiber des globalen Marktes für Hepatitis-B-Infektionen und führt zu einer steigenden Nachfrage nach wirksamen Diagnoseinstrumenten, Impfstoffen und antiviralen Behandlungen. Da Hepatitis B eine Hauptursache für Lebererkrankungen wie Leberzirrhose und Leberkrebs ist, haben die weltweit steigenden Infektionsraten, insbesondere in Regionen mit unzureichender Gesundheitsinfrastruktur, den Bedarf an Präventions-, Management- und Behandlungsmöglichkeiten erhöht. Die Ausbreitung chronischer Hepatitis-B-Infektionen, insbesondere in Ländern mit niedrigem und mittlerem Einkommen, in denen Aufklärungs- und Impfprogramme weniger etabliert sind, verstärkt den Markt für Immunisierungen, antivirale Therapien und fortschrittliche Diagnoseverfahren zusätzlich. Pharmaunternehmen investieren zudem in die Forschung und Entwicklung neuerer, effizienterer antiviraler Medikamente und Impfstoffe, um den sich entwickelnden Virusstämmen entgegenzuwirken. Der Anstieg sowohl akuter als auch chronischer Fälle, insbesondere in Hochrisikogruppen, setzt die Gesundheitssysteme unter Druck, in verbesserte Diagnostik, Impfstoffe und antivirale Therapien zu investieren. Insgesamt treibt die zunehmende Belastung durch Hepatitis-B-Infektionen die Nachfrage nach therapeutischen und präventiven Lösungen voran und befeuert so das Marktwachstum.
Vollständigen Bericht abrufen unter https://www.databridgemarketresearch.com/reports/global-hepatitis-b-infection-market
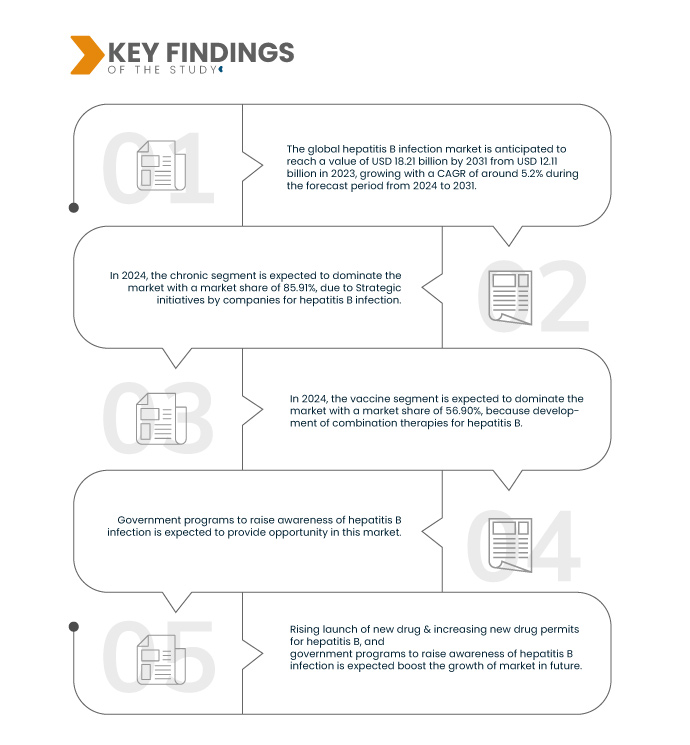
Data Bridge Market Research analysiert, dass der globale Markt für Hepatitis-B-Behandlungen von 12,11 Milliarden US-Dollar im Jahr 2023 auf 18,21 Milliarden US-Dollar im Jahr 2031 anwachsen dürfte, was einem CAGR von 5,2 % im Prognosezeitraum von 2024 bis 2031 entspricht.
Wichtigste Ergebnisse der Studie
Technologische Fortschritte in der Diagnostik
Technologische Fortschritte in der Diagnostik spielen eine entscheidende Rolle für den globalen Markt für Hepatitis-B-Infektionen, da sie die Genauigkeit, Geschwindigkeit und Zugänglichkeit der Hepatitis-B-Erkennung verbessern. Innovationen wie Next-Generation-Sequencing (NGS), Polymerase-Kettenreaktion (PCR) und Schnelltests haben die Fähigkeit verbessert, Hepatitis-B-Infektionen in früheren Stadien, auch in asymptomatischen Fällen, zu erkennen und so eine rechtzeitige Intervention und Behandlung zu ermöglichen. Diese Technologien ermöglichen eine präzisere Quantifizierung der Viruslast, personalisierte Behandlungsansätze und eine bessere Überwachung des Krankheitsverlaufs. Die Entwicklung von Point-of-Care-Diagnosetools hat zudem den Zugang zu Tests in abgelegenen und ressourcenarmen Regionen, in denen Hepatitis B häufiger vorkommt, verbessert und die Marktnachfrage deutlich gesteigert. Darüber hinaus revolutionieren fortschrittliche Diagnoseplattformen mit integrierter künstlicher Intelligenz (KI) und maschinellem Lernen (ML) die Datenanalyse und ermöglichen es medizinischem Fachpersonal, schnellere und fundiertere Entscheidungen zu treffen. Da sich diese Technologien weiterentwickeln und kostengünstiger werden, tragen sie zu einer zunehmenden Akzeptanz im Gesundheitswesen bei und treiben den globalen Markt für Hepatitis-B-Infektionen weiter voran.
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2024 bis 2031
|
Basisjahr
|
2023
|
Historisches Jahr
|
2022 (Anpassbar auf 2016 – 2021)
|
Quantitative Einheiten
|
Umsatz in Milliarden USD
|
Abgedeckte Segmente
|
Typ (chronisch und akut), Behandlung (Impfstoff, antivirale Medikamente , immunmodulierende Medikamente und Operation)
|
Abgedeckte Länder
|
USA, Kanada und Mexiko, China, Indien, Japan, Südkorea, Singapur, Thailand, Malaysia, Indonesien, Australien, Vietnam, Neuseeland, Taiwan, Philippinen und übriger Asien-Pazifik-Raum, Deutschland, Großbritannien, Frankreich, Italien, Spanien, Russland, Niederlande, Schweiz, Belgien, Schweden, Dänemark, Norwegen, Finnland, Polen, Türkei und übriges Europa, Südafrika, Saudi-Arabien, Ägypten, Kuwait, Katar, Vereinigte Arabische Emirate, Oman, Bahrain und übriger Naher Osten und Afrika, Brasilien, Argentinien und übriges Südamerika
|
Abgedeckte Marktteilnehmer
|
Gilead Sciences, Inc. (USA), GlaxoSmithKline plc (Großbritannien), Dynavax Technologies (USA), F. Hoffmann-La Roche Ltd (Schweiz), Bristol-Myers Squibb Company (USA), Merck Sharp and Dohme Corp. (USA), Novartis AG (Schweiz), Arrowhead Pharmaceuticals Inc. (USA), Arbutus Biopharma (Kanada), Teva Pharmaceuticals, Inc. (Israel), Zydus Pharmaceuticals (Indien), Aurobindo Pharma (Indien), Lupin Pharmaceuticals, Inc. (Indien) und andere
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
|
Segmentanalyse
- Auf der Grundlage des Typs ist der Markt in chronische und akute unterteilt
Im Jahr 2024 wird das chronische Segment des Typsegments voraussichtlich den Markt dominieren
Im Jahr 2024 wird das chronische Segment voraussichtlich mit einem Marktanteil von 85,91 % den Markt dominieren, da die Behandlung chronischer Hepatitis-B-Infektionen langwierig und komplex ist. Im Gegensatz zur akuten Hepatitis B, die oft von selbst abheilt, können chronische Infektionen jahrelang bestehen bleiben und das Risiko schwerer Leberkomplikationen wie Leberzirrhose, Leberversagen und hepatozellulärem Karzinom erheblich erhöhen.
- Auf der Grundlage der Behandlung ist der Markt in Impfstoffe, antivirale Medikamente, Immunmodulatoren und Operationen unterteilt
Im Jahr 2024 wird das Impfstoffsegment des Behandlungssegments voraussichtlich den globalen Markt für Hepatitis-B-Behandlungen dominieren
Im Jahr 2024 wird das Impfstoffsegment voraussichtlich mit einem Marktanteil von 56,90 % den Markt dominieren, da die Nachfrage nach elektronischen Komponenten für die Automobilindustrie steigt.
Hauptakteure
Laut einer Analyse von Data Bridge Market Research sind Gilead Sciences, Inc. (USA), GlaxoSmithKline plc (Großbritannien), Dynavax Technologies (USA), F. Hoffmann-La Roche Ltd (Schweiz) und Bristol-Myers Squibb Company die wichtigsten Marktteilnehmer auf diesem Markt.
Marktentwicklung
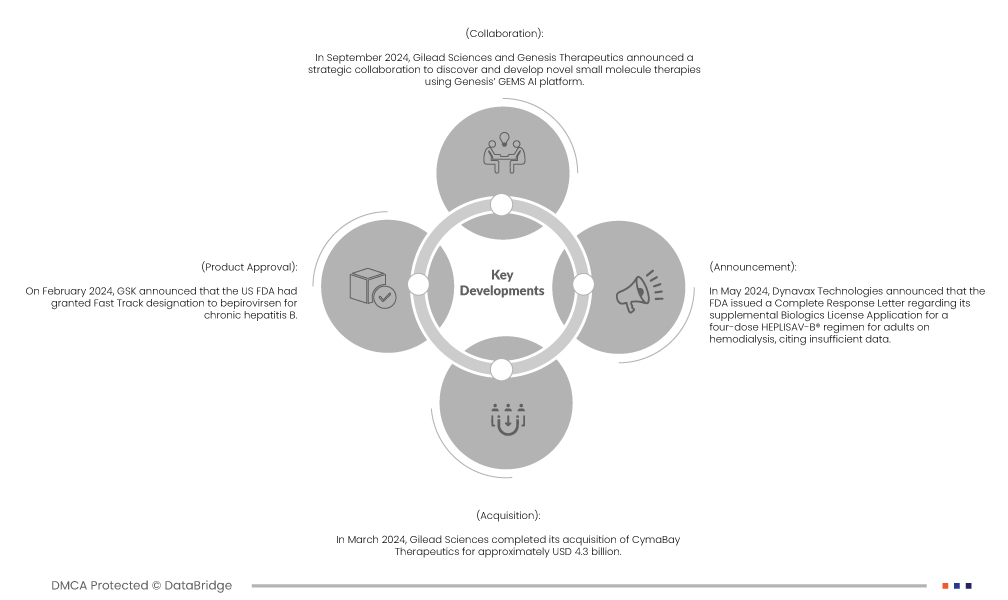
- Im Februar 2024 gab GSK bekannt, dass die US-amerikanische FDA Bepirovirsen den Fast-Track-Status für chronische Hepatitis B erteilt hatte. Dieser Status verdeutlichte den ungedeckten Bedarf an wirksamen Behandlungen und unterstützte die laufenden Phase-III-Studien für das Prüfpräparat.
- Im März 2024 schloss Gilead Sciences die Übernahme von CymaBay Therapeutics für rund 4,3 Milliarden US-Dollar ab. Mit dieser im Februar 2024 angekündigten Übernahme wurde CymaBays Prüfpräparat Seladelpar in Gileads Portfolio für Lebererkrankungen integriert.
- Im Mai 2024 gab Dynavax Technologies bekannt, dass die FDA einen vollständigen Antwortbrief zu ihrem ergänzenden Zulassungsantrag für ein vierdosiges HEPLISAV-B®-Regime für erwachsene Hämodialysepatienten herausgegeben hat. Der Antrag wurde mit der Begründung unzureichender Daten beantwortet. Die zugelassene Indikation für HEPLISAV-B bleibt unverändert, während die Europäische Kommission das Regime im Oktober 2023 genehmigte.
- Im September 2024 gaben Gilead Sciences und Genesis Therapeutics eine strategische Zusammenarbeit zur Erforschung und Entwicklung neuartiger niedermolekularer Therapien mithilfe der GEMS-KI-Plattform von Genesis bekannt. Gilead erhielt im Rahmen dieser Partnerschaft die exklusiven Rechte zur Entwicklung und Vermarktung von Produkten.
Geografisch betrachtet sind dies die folgenden Länder, die in diesem Marktbericht abgedeckt sind: USA, Kanada und Mexiko, China, Indien, Japan, Südkorea, Singapur, Thailand, Malaysia, Indonesien, Australien, Vietnam, Neuseeland, Taiwan, die Philippinen und der Rest des asiatisch-pazifischen Raums, Deutschland, Großbritannien, Frankreich, Italien, Spanien, Russland, die Niederlande, die Schweiz, Belgien, Schweden, Dänemark, Norwegen, Finnland, Polen, die Türkei und der Rest Europas, Südafrika, Saudi-Arabien, Ägypten, Kuwait, Katar, die Vereinigten Arabischen Emirate, Oman, Bahrain und der Rest des Nahen Ostens und Afrikas, Brasilien, Argentinien und der Rest Südamerikas.
Laut Marktforschungsanalyse von Data Bridge :
Der asiatisch-pazifische Raum wird voraussichtlich die dominierende und am schnellsten wachsende Region im globalen Markt für Hepatitis-B-Behandlungen sein
Aufgrund der technologischen Fortschritte in der Diagnostik wird erwartet, dass der asiatisch-pazifische Raum die dominierende und am schnellsten wachsende Region wird.
Für detailliertere Informationen zum globalen Marktbericht zur Hepatitis-B-Behandlung klicken Sie hier – https://www.databridgemarketresearch.com/reports/global-hepatitis-b-infection-market