سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية، حسب نوع المنتج (الأدوات والمواد الاستهلاكية والملحقات)، نوع الاختبار (اختبار التصوير، الخزعة، اختبار الدم، أخرى)، نوع السرطان (سرطان حليمي، سرطان جرابي، أخرى)، المراحل (المرحلة الأولى، المرحلة الثانية، المرحلة الثالثة، المرحلة الرابعة)، الفئة العمرية (أقل من 21، 21-29، 30-65، 65 وما فوق)، المستخدم النهائي (المستشفى، المختبرات المرتبطة، مختبرات التشخيص المستقلة، مراكز التصوير التشخيصي، معاهد أبحاث السرطان، وغيرها)، قناة التوزيع (العطاء المباشر، مبيعات التجزئة) - اتجاهات الصناعة والتوقعات حتى عام 2030.
تحليل ورؤى حول سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية
لقد أدى زيادة الوعي بسرطان الغدة الدرقية إلى تعزيز الطلب في السوق. كما يساهم ارتفاع الإنفاق على الرعاية الصحية لتحسين الخدمات الصحية في نمو السوق. يركز اللاعبون الرئيسيون في السوق على إطلاق خدمات مختلفة والموافقات عليها خلال هذه الفترة الحاسمة. بالإضافة إلى ذلك، يساهم زيادة عمليات وتقنيات التشخيص المحسنة أيضًا في زيادة الطلب على اختبارات تشخيص سرطان الغدة الدرقية.
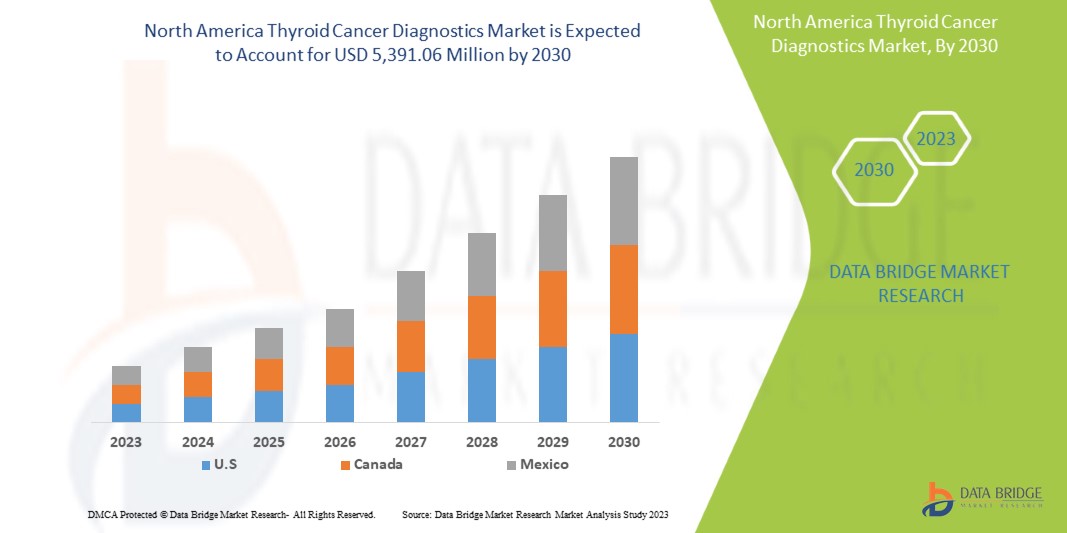
من المتوقع أن ينمو سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية في الفترة المتوقعة من 2023 إلى 2030. تحلل شركة Data Bridge Market Research أن السوق ينمو بمعدل نمو سنوي مركب بنسبة 6.4٪ في الفترة المتوقعة من 2023 إلى 2030 ومن المتوقع أن يصل إلى 5،391.06 مليون دولار أمريكي بحلول عام 2030.
|
تقرير القياس |
تفاصيل |
|
فترة التنبؤ |
2023 إلى 2030 |
|
سنة الأساس |
2022 |
|
سنوات تاريخية |
2021 (قابلة للتخصيص حتى 2020-2015) |
|
وحدات كمية |
الإيرادات بالملايين من الدولارات الأمريكية |
|
القطاعات المغطاة |
حسب نوع المنتج (الأدوات والمواد الاستهلاكية والملحقات)، نوع الاختبار (اختبار التصوير، الخزعة، اختبار الدم، أخرى)، نوع السرطان (سرطان حليمي، سرطان جرابي، أخرى)، المراحل (المرحلة الأولى، المرحلة الثانية، المرحلة الثالثة، المرحلة الرابعة)، الفئة العمرية (أقل من 21، 21-29، 30-65، 65 وما فوق)، المستخدم النهائي (المستشفى، المختبرات المرتبطة، مختبرات التشخيص المستقلة، مراكز التصوير التشخيصي، معاهد أبحاث السرطان، وغيرها)، قناة التوزيع (العطاء المباشر، مبيعات التجزئة) |
|
الدول المغطاة |
الولايات المتحدة وكندا والمكسيك |
|
الجهات الفاعلة في السوق المشمولة |
Canon Inc.، FUJIFILM Holdings Corporation، F. Hoffmann-La Roche Ltd، Quest Diagnostics Incorporated، Illumina، Koninklijke Philips NV، Thermo Fisher Scientific Inc.، Siemens Healthcare GmbH، Abbott، General Electric Company، BD، QIAGEN، DIASORIN SPA، Merck KGaA، Hologic، Myriad Genetics Inc.، BIOMERIEUX، FONAR Corp.، Time Medical Holding.، PlexBio.، MinFound Medical Systems Co.، Ltd، Medonica Co. LTD، Beijing O&D Biotech Co.، Ltd، وSternMed GmbH. من بين شركات أخرى |
تعريف السوق
سرطان الغدة الدرقية هو نوع من السرطان يبدأ في الغدة الدرقية. يبدأ السرطان عندما تبدأ الخلايا في النمو خارج نطاق السيطرة. تفرز الغدة الدرقية هرمونات تساعد في تنظيم عملية التمثيل الغذائي ومعدل ضربات القلب وضغط الدم ودرجة حرارة الجسم. تقع الغدة الدرقية في الجزء الأمامي من الرقبة، أسفل غضروف الغدة الدرقية (تفاحة آدم). في معظم الناس، لا يمكن رؤية الغدة الدرقية أو الشعور بها. لها شكل يشبه الفراشة، مع فصين - الفص الأيمن والفص الأيسر - متصلين بقطعة ضيقة من الغدة تسمى البرزخ.
ديناميكيات سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية
السائقين
ارتفاع معدل الإصابة وانتشار عقيدات الغدة الدرقية والسرطان
عقيدات الغدة الدرقية هي نمو غير عادي (كتلة) لخلايا الغدة الدرقية في الغدة الدرقية. في بعض الأحيان، تبدأ الأنسجة الرخوة الطبيعية للغدة الدرقية في النمو، مما يتسبب في تكوين هذه العقيدات. يزداد حدوث العقيدات مع تقدم العمر وخاصة عند النساء، وخاصة أولئك الذين يعانون من نقص اليود وبعد التعرض للإشعاع. على الرغم من أن المضاعفات الإضافية لهذه العقيدات هي سرطان الغدة الدرقية، فإن فرص تحول عقيدات الغدة الدرقية إلى سرطان الغدة الدرقية منخفضة. وفقًا لمقال نُشر في NCBI بعنوان "خطر الإصابة بالأورام الخبيثة في عقيدات الغدة الدرقية التي يبلغ حجمها 4 سم أو أكبر" في عام 2017، فإن حدوث السرطان بعد العقيدات موجود في أقل من 5٪ من إجمالي حالات العقيدات. علاوة على ذلك، فإن عقيدات الغدة الدرقية موجودة في التاريخ العائلي وفي الأشخاص الذين يكون تناول اليود لديهم منخفضًا.
اختبارات تشخيصية لسرطان الغدة الدرقية
الموجات فوق الصوتية للغدة الدرقية هي صورة موجة صوتية للغدة الدرقية يتم التقاطها بواسطة أداة يدوية وترجمتها إلى صورة ثنائية الأبعاد على شاشة. تُستخدم في تشخيص الأورام أو الأكياس أو تضخم الغدة الدرقية وهي إجراء غير مؤلم ولا ينطوي على مخاطر. تُستخدم هذه الاختبارات لتقييم الشذوذ البنيوي، في حين تُستخدم اختبارات الدم التي تقيس مستويات TSH وT4 وT3 لفحص المتغيرات الوظيفية. تُستخدم خزعة الإبرة الدقيقة في الحالات المشتبه بها لتحديد ما إذا كان الورم حميدًا أم خبيثًا. علاوة على ذلك، فإن إدخال الاختبارات الجزيئية والتشخيص الوراثي يغذي المشهد التشخيصي في سوق تشخيص سرطان الغدة الدرقية.
زيادة الوعي بسرطان الغدة الدرقية
لقد أدى الوعي المتزايد بسرطان الغدة الدرقية إلى زيادة الطلب على الكشف عن السرطان في الوقت المناسب، مما أدى إلى نمو السوق.
يعد سرطان الغدة الدرقية أحد الأسباب الرئيسية لارتفاع معدلات الوفيات بين سكان الولايات المتحدة في جميع أنحاء العالم، مما يؤدي إلى نمو السوق على مدى السنوات الخمس المقبلة. يعد التعرض للإشعاع والتاريخ العائلي لمشاكل الغدة الدرقية من عوامل الخطر الرئيسية لسرطان الغدة الدرقية. يتم تشخيص إصابة النساء بسرطان الغدة الدرقية أكثر بكثير من الرجال.
يقيد
ارتفاع تكلفة إجراءات التشخيص
أصبحت تشخيصات السرطان مكلفة بشكل متزايد بسبب العدد المتزايد من مرضى سرطان الغدة الدرقية وارتفاع أسعار الأجهزة الطبية. تلعب الأجهزة التكنولوجية الحديثة المستخدمة في تشخيص السرطان أيضًا دورًا مهمًا في ارتفاع أسعار تشخيص السرطان والدقة العالية في توفير تشخيص نهائي للسرطان في عقيدات الغدة الدرقية. لذلك، فإن التكلفة العالية لإجراءات تشخيص سرطان الغدة الدرقية تعيق نمو السوق.
أصبحت المنتجات أو الأجهزة التشخيصية المستخدمة في الكشف عن السرطان متقدمة ولكن إلى جانب ذلك فإن إجراءات تشخيص السرطان مكلفة أيضًا، مما يعيق نمو سوق تشخيص سرطان الغدة الدرقية لأن الأجهزة المستخدمة في عملية تشخيص السرطان أصبحت أكثر تكلفة مما يؤدي إلى زيادة تكلفة إجراءات التشخيص. وبالتالي فإن التكلفة المرتفعة لتشخيص السرطان تعمل كقيد لسوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية.
تلف الأنسجة بسبب التعرض للإشعاع العالي من اختبارات التصوير
يؤدي التعرض لجرعات عالية من الإشعاع إلى تلف الأنسجة بشكل كبير ويزيد من فرصة إصابة الشخص بالسرطان لاحقًا. وعلى الرغم من أهمية وضع هذا الخطر في سياقه، إلا أن جرعات الإشعاع الصغيرة المستخدمة في اختبارات التصوير قد تزيد بشكل طفيف من خطر إصابة الشخص بالسرطان. وتختلف كمية الإشعاع التي يتلقاها الشخص حسب نوع الاختبار، والمنطقة المعرضة من جسمه، وحجم جسمه، وعمره، وجنسه، من بين أمور أخرى. يجب إجراء فحوصات التصوير التي تستخدم الإشعاع فقط عند الضرورة لأن التعرض للإشعاع من جميع المصادر يمكن أن يتراكم على مدار العمر ويمكن أن يزيد من فرصة الإصابة بالسرطان. يمكن أيضًا استخدام إجراءات التصوير الأخرى مثل الموجات فوق الصوتية أو التصوير بالرنين المغناطيسي في كثير من الأحيان.
فرص
ارتفاع الإنفاق على الرعاية الصحية لتشخيص وعلاج السرطان
في جميع أنحاء العالم، تتزايد أنشطة البحث والتطوير بسبب الإنفاق على الصحة العامة مع الأداء الاقتصادي، في حين تحتل صناعة الرعاية الصحية المرتبة الثانية بين جميع الصناعات عندما يتعلق الأمر بالمبلغ الذي يتم إنفاقه على الرعاية الصحية. يمكن أن يؤدي ارتفاع الإنفاق على الرعاية الصحية إلى توفير فرص أفضل للبحث والتطوير. ومن المتوقع أن يرتفع الطلب على تشخيص سرطان المبيض.
كما تساعد زيادة الإنفاق على الرعاية الصحية لعلاج السرطان المرضى على الحصول على تشخيصات وعلاجات متقدمة خالية من المتاعب للتعافي السريع. يتكون الإنفاق على الرعاية الصحية من مدفوعات من الجيب (يدفع الأشخاص مقابل رعايتهم بأنفسهم)، والإنفاق الحكومي، والمصادر، بما في ذلك التأمين الصحي وأنشطة المنظمات غير الحكومية. وبسبب هذا الإنفاق المتزايد على الرعاية الصحية لعلاج السرطان، فإنه يعمل كفرصة لنمو السوق.
التحديات
إطار تنظيمي صارم للموافقة على منتجات تشخيص السرطان وتسويقها
لقد أثبتت اللوائح الصارمة التي تحكم الموافقة على أي منتج وتسويقه في السوق أنها تشكل أحد التحديات الرئيسية التي تواجه الشركات المصنعة لمنتجات تشخيص السرطان في الولايات المتحدة ومنطقة أوروبا. فلكل دولة لوائح وهيئة مختلفة للإجراءات التنظيمية.
المسارات التنظيمية المحتملة إلزامية للحصول على الموافقة أو الموافقة أو قبول التوقيعات المعقدة من قبل إدارة الغذاء والدواء الأمريكية (FDA). تتضمن المسارات التنظيمية اللوائح المعمول بها على أجهزة التشخيص المختبري (IVD)، بما في ذلك أجهزة التشخيص المصاحبة، وإمكانية وضع العلامات كتشخيص تكميلي، وبرنامج تأهيل المؤشرات الحيوية.
التطورات الأخيرة
- في أغسطس 2022، أعلنت شركة F. Hoffmann-La Roche Ltd عن إطلاق نظام Digital LightCycler، وهو أول نظام تفاعل بوليميراز متسلسل رقمي من Roche. يكتشف هذا النظام من الجيل التالي الأمراض وهو مصمم لقياس كميات ضئيلة من أهداف DNA وRNA المحددة التي لا يمكن اكتشافها عادةً بواسطة طرق تفاعل البوليميراز المتسلسل التقليدية. وقد ساعد هذا الشركة على زيادة حضورها في السوق في أمريكا الشمالية
- في مايو 2022، قدمت شركة Thermo Fisher Scientific Inc.، الشركة الرائدة عالميًا في خدمة العلوم، مجهر Thermo Scientific Glacios 2 Cryo-Transmission Electron Microscope (Cryo-TEM)، وهو مجهر قوي مزود بقدرات أتمتة جديدة وتصوير عالي الدقة مصمم لمساعدة الباحثين في مجال المجهر الإلكتروني بالتبريد (cryo-EM) من مختلف مستويات الخبرة في تسريع اكتشاف الأدوية القائمة على البنية. قد تمكن هذه الطريقة المتقدمة والسريعة والفعّالة من حيث التكلفة لتصميم الأدوية العملاء من تسريع وتيرة البحث عن الاضطرابات المنهكة مثل مرض الزهايمر ومرض باركنسون ومرض هنتنغتون، بالإضافة إلى البحث عن السرطان والطفرات الجينية
نطاق سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية
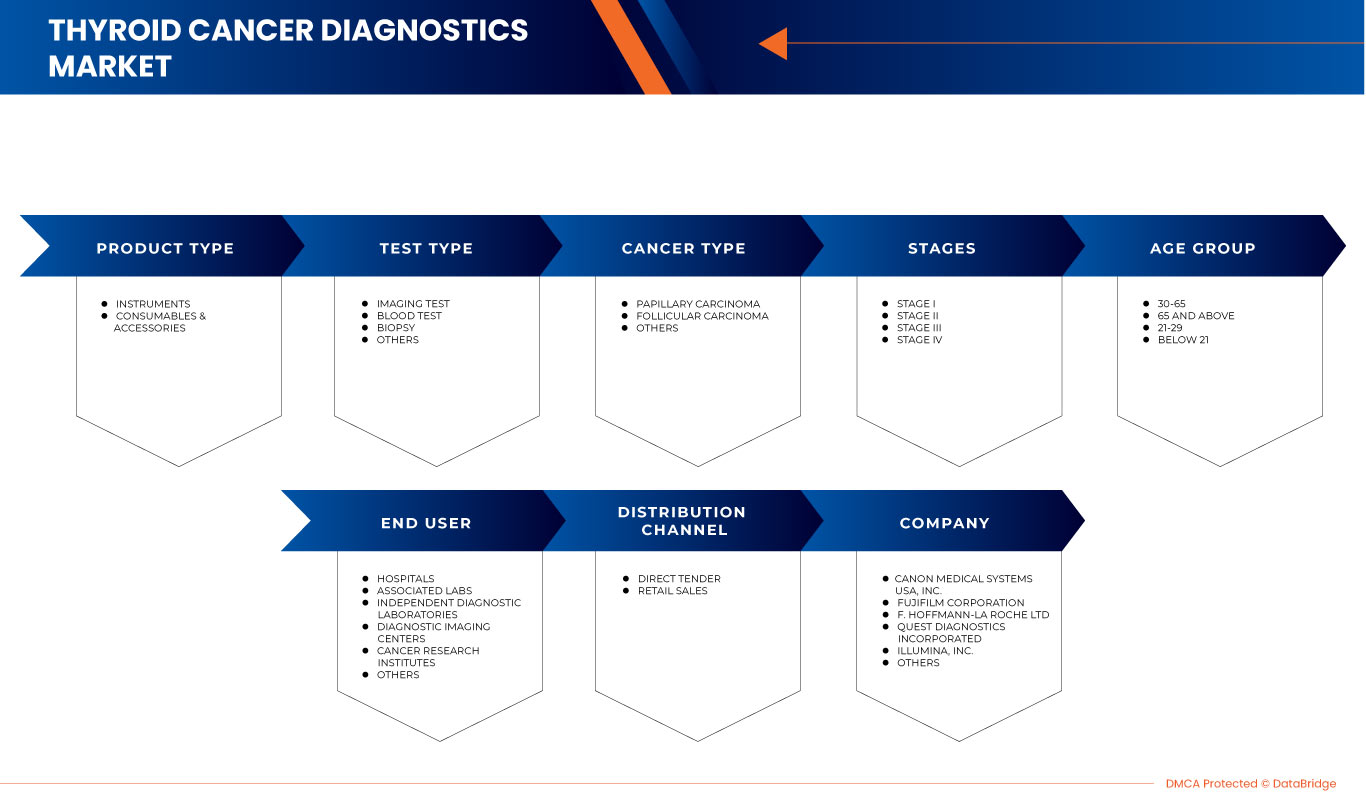
يتم تقسيم سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية إلى نوع المنتج ونوع الاختبار ونوع السرطان والمراحل والفئة العمرية والمستخدم النهائي وقناة التوزيع. سيساعدك النمو بين هذه القطاعات على تحليل قطاعات النمو الضئيلة في الصناعات وتزويد المستخدمين بنظرة عامة قيمة على السوق ورؤى السوق لاتخاذ قرارات استراتيجية لتحديد تطبيقات السوق الأساسية.
نوع المنتج
- الآلات الموسيقية
- المواد الاستهلاكية والملحقات
على أساس نوع المنتج، يتم تقسيم سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية إلى أدوات ومواد استهلاكية وملحقات.
نوع الاختبار
- اختبار التصوير
- فحص الدم
- الخزعة
- آحرون
على أساس نوع الاختبار، يتم تقسيم سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية إلى اختبار التصوير، واختبار الدم، والخزعة وغيرها.
نوع السرطان
- سرطان حليمي
- سرطان الجريبات
- آحرون
على أساس نوع السرطان، يتم تقسيم سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية إلى سرطان حليمي، وسرطان جرابي، وغيرها.
مراحل
- المرحلة الأولى
- المرحلة الثانية
- المرحلة الثالثة
- المرحلة الرابعة
On the basis of stages, the North America thyroid cancer diagnostics market is segmented into stage I, stage II, stage III and stage IV.
Age Group
- 30-65
- 65 and above
- 21-29
- Below 21
On the basis of age group, the North America thyroid cancer diagnostics market is segmented into 30-65, 65 and above, 21-29, and below 21.
End User
- Hospitals
- Associated Labs
- Independent Diagnostic Laboratories
- Diagnostic Imaging Centers
- Cancer Research Institutes
- Others
On the basis of end user, the North America thyroid cancer diagnostics market is segmented into hospitals, associated labs, independent diagnostic laboratories, diagnostic imaging centers, cancer research institutes, and others.
Distribution Channel
- Direct Tender
- Retail Sales
On the basis of distribution channel, the North America thyroid cancer diagnostics market is segmented into direct tender and retail sales.
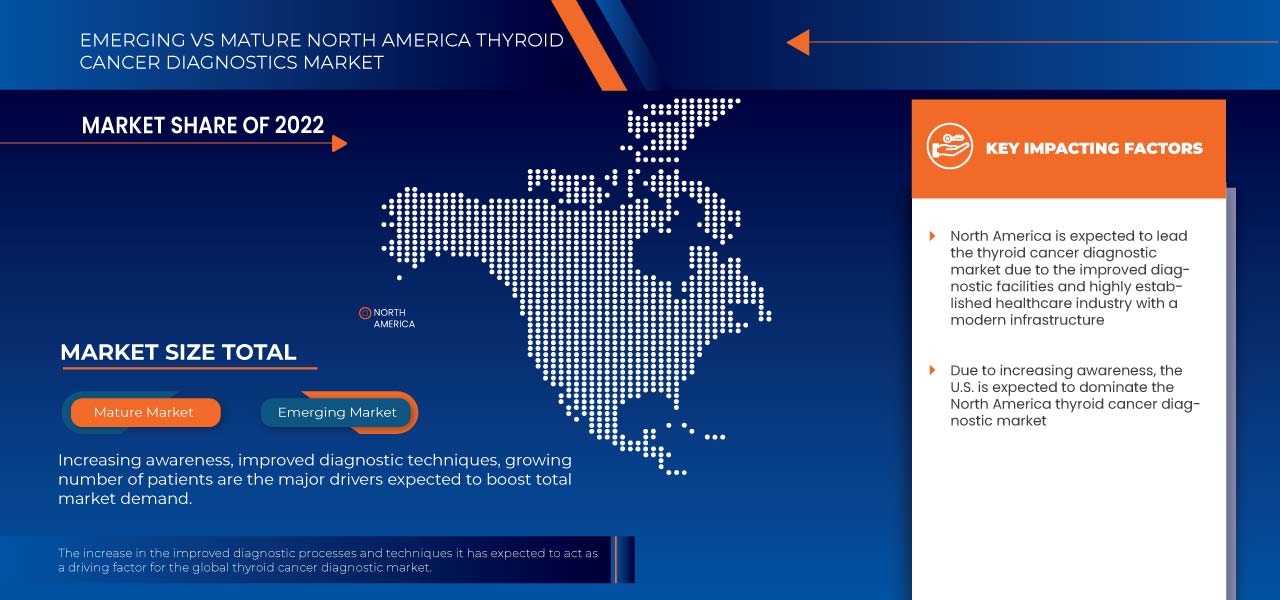
North America Thyroid Cancer Diagnostics Market Regional Analysis/Insights
The North America thyroid cancer diagnostics market is analysed, and market size insights and trends are provided by country, product type, test type, cancer type, stages, age group, end user, and distribution channel, as referenced above.
The countries covered in this market report are U.S., Canada, and Mexico.
The U.S. is expected to dominate the North America thyroid cancer diagnostics market in terms of market share and revenue and will continue to flourish its dominance during the forecast period. This is due to rising thyroid cancer diagnostic tests.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of North American brands and the challenges faced due to competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Thyroid Cancer Diagnostics Market Share Analysis
North America thyroid cancer diagnostics market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the North America thyroid cancer diagnostics market.
بعض اللاعبين الرئيسيين العاملين في سوق تشخيص سرطان الغدة الدرقية في أمريكا الشمالية هم Canon Inc. و FUJIFILM Holdings Corporation و F. Hoffmann-La Roche Ltd و Quest Diagnostics Incorporated و Illumina و Koninklijke Philips NV و Thermo Fisher Scientific Inc. و Siemens Healthcare GmbH و Abbott و General Electric Company و BD و QIAGEN و DIASORIN SPA و Merck KGaA و Hologic و Myriad Genetics Inc. و BIOMERIEUX و FONAR Corp. و Time Medical Holding. و PlexBio. و MinFound Medical Systems Co.، Ltd و Medonica Co. LTD و Beijing O&D Biotech Co.، Ltd. و SternMed GmbH. من بين آخرين.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GROWTH STRATEGIES ADOPTED BY KEY MARKET PLAYERS
5 EPIDEMIOLOGY
6 REGULATORY FRAMEWORK OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
6.1 REGULATORY SCENARIO IN THE U.S.
6.2 REGULATORY SCENARIO IN AUSTRALIA
6.3 REGULATORY SCENARIO IN JAPAN
6.4 REGULATORY SCENARIO IN CHINA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISING INCIDENCE AND PREVALENCE OF THYROID NODULES AND CANCER
7.1.2 RISING THYROID CANCER DIAGNOSTIC TESTS
7.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.1.4 RISING AWARENESS TOWARDS THYROID CANCER
7.2 RESTRAINTS
7.2.1 HIGH COST OF DIAGNOSTICS PROCEDURE
7.2.2 TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
7.3 OPPORTUNITIES
7.3.1 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.3.2 RISING OBESE POPULATION
7.4 CHALLENGES
7.4.1 STRINGENT REGULATORY FRAMEWORK FOR THE APPROVAL AND COMMERCIALIZATION OF CANCER DIAGNOSTIC PRODUCTS
7.4.2 LACK OF SKILLED AND CERTIFIED EXPERTISE
8 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 INSTRUMENTS
8.2.1 PATHOLOGY BASED INSTRUMENTS
8.2.1.1 PCR INSTRUMENTS
8.2.1.2 SLIDE STAINING SYSTEMS
8.2.1.3 TISSUE PROCESSING SYSTEMS
8.2.1.4 CELL PROCESSORS
8.2.1.5 OTHER PATHOLOGY-BASED INSTRUMENTS
8.2.2 IMAGING INSTRUMENTS
8.2.2.1 ULTRASOUND SYSTEMS
8.2.2.2 CT SYSTEMS
8.2.2.3 MRI SYSTEMS
8.2.2.4 OTHERS
8.2.3 BIOPSY INSTRUMENTS
8.2.3.1 NEEDLE BIOPSY
8.2.3.2 ENDOSCOPIC BIOPSY
8.2.3.3 CORE BIOPSY
8.2.3.4 OTHERS
8.2.4 OTHERS
8.3 CONSUMABLES & ACCESSORIES
8.3.1 KITS
8.3.1.1 PCR KITS
8.3.1.2 DNA POLYMERASE KITS
8.3.1.3 NUCLEIC ACID ISOLATION KITS
8.3.1.4 OTHERS
8.3.2 REAGENTS
8.3.2.1 ASSAYS
8.3.2.2 BUFFERS
8.3.2.3 PRIMERS
8.3.2.4 OTHERS
8.3.3 PROBES
8.3.4 OTHER CONSUMABLES
9 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.2 MRI
9.2.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
9.2.4 OTHERS
9.3 BLOOD TEST
9.3.1 BLOOD CHEMISTRY TESTS
9.3.2 COMPLETE BLOOD COUNT (CBC)
9.3.3 OTHERS
9.4 BIOPSY
9.4.1 NEEDLE BIOPSY
9.4.2 BRONCHOSCOPY BIOPSY
9.4.3 CORE BIOPSY
9.4.4 OTHERS
9.5 OTHERS
10 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 PAPILLARY CARCINOMA
10.3 FOLLICULAR CARCINOMA
10.4 OTHERS
11 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES
11.1 OVERVIEW
11.2 STAGE I
11.3 STAGE II
11.4 STAGE III
11.5 STAGE IV
12 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP
12.1 OVERVIEW
12.2 30-65
12.3 65 AND ABOVE
12.4 21-29
12.5 BELOW 21
13 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.3 ASSOCIATED LABS
13.4 INDEPENDENT DIAGNOSTIC LABORATORIES
13.5 DIAGNOSTIC IMAGING CENTERS
13.6 CANCER RESEARCH INSTITUTES
13.7 OTHERS
14 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL SALES
15 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 CANON INC.
18.1.1 COMPANY SNAPSHOT
18.1.2 COMPANY SHARE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 FUJIFILM CORPORATION
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENT
18.3 F. HOFFMANN-LA ROCHE LTD
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 QUEST DIAGNOSTICS INCORPORATED
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 ILLUMINA, INC.
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBOTT
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 BD
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 BEIJING O&D BIOTECH CO., LTD.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 BIOMÉRIEUX SA
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENTS
18.1 DIASORIN S.P.A.
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 FONAR CORP.
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENTS
18.12 GENERAL ELECTRIC
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 HOLOGIC INC.
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 KONINKLIJKE PHILIPS N.V.
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 MERCK KGAA.
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MEDONICA CO. LTD
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MINFOUND MEDICAL SYSTEMS CO. LTD
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENTS
18.18 MYRIAD GENETICS, INC.
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENTS
18.19 PLEXBIO.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENTS
18.2 QIAGEN
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 STERNMED GMBH
18.21.1 COMPANY SNAPSHOT
18.21.2 PRODUCT PORTFOLIO
18.21.3 RECENT DEVELOPMENTS
18.22 SIEMENS HEALTHCARE GMBH
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENT
18.23 TIME MEDICAL HOLDING.
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 THERMO FISHER SCIENTIFIC INC.
18.24.1 COMPANY SNAPSHOT
18.24.2 REVENUE ANALYSIS
18.24.3 PRODUCT PORTFOLIO
18.24.4 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 2 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA PAPILLARY CARCINOMA IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA FOLLICULAR CARCINOMA IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA STAGE I IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA STAGE II IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA STAGE III IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA STAGE IV IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA 30-65 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA 65 AND ABOVE IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA 21-29 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA BELOW 21 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA HOSPITALS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA ASSOCIATED LABS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA INDEPENDENT DIAGNOSTIC LABORATORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA DIAGNOSTIC IMAGING CENTERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA CANCER RESEARCH INSTITUTES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA DIRECT TENDER IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA RETAIL SALES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 61 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 U.S. INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 U.S. PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 U.S. IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 65 U.S. BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 66 U.S. CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 67 U.S. KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 68 U.S. REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 69 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 U.S. IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 U.S. BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 U.S. BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 74 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 75 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 76 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 78 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 CANADA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 CANADA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 CANADA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 CANADA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 CANADA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 CANADA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 85 CANADA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 86 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 CANADA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 88 CANADA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 89 CANADA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 90 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 91 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 92 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 93 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 94 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 95 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 MEXICO INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 MEXICO PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 MEXICO IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 MEXICO BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 MEXICO CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 MEXICO KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 MEXICO REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 103 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 MEXICO IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 MEXICO BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 MEXICO BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 108 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 109 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 110 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 111 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN THE AWARENESS ABOUT THYROID CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET IN THE FORECAST PERIOD
FIGURE 12 PRODUCT TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 19 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 23 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, 2022
FIGURE 27 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, LIFELINE CURVE
FIGURE 30 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, 2022
FIGURE 31 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 34 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 35 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 39 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 42 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 43 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 44 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 45 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 46 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: PRODUCT TYPE (2023-2030)
FIGURE 47 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.