سوق علاج الورم الأرومي الدبقي متعدد الأشكال في أمريكا الشمالية، حسب النوع (أولي (جديد)، ثانوي)، العلاج (الجراحة، العلاج الإشعاعي ، الأدوية)، نوع المريض (بالغ، مسن ، طفل)، نوع الدواء (الأدوية الجنيسة، ذات العلامة التجارية)، طريق الإدارة (الحقن، الفموي، أخرى)، المستخدم النهائي (المستشفيات، العيادات، الرعاية الصحية المنزلية، أخرى)، قناة التوزيع (صيدلية المستشفى، صيدلية التجزئة، صيدلية الإنترنت، أخرى) - اتجاهات الصناعة والتوقعات حتى عام 2029.
تحليل السوق والرؤى
الورم الأرومي الدبقي متعدد الأشكال (GBM) هو ورم خبيث من الدرجة الرابعة وفقًا لمنظمة الصحة العالمية مع تمايز نجمي. باعتباره أحد أكثر مداخل الأورام شيوعًا التي يتم تشخيصها سريريًا في الجهاز العصبي المركزي (CNS)، كانت هناك مجموعة واسعة من التقارير التاريخية لوصف وتطور الأفكار المتعلقة بهذه الأورام. تم تقديم التقارير المسجلة الأولى للأورام الدبقية في التقارير العلمية البريطانية، بواسطة بيرنز في عام 1800 وفي عام 1804 بواسطة أبيرنيتي، مع إعطاء أول وصف هيستومورفولوجي شامل في عام 1865 بواسطة رودولف فيرشو. في عام 1926، أعطى بيرسيفال بيلي وهارفي كوشينج الأساس للتصنيف الحديث للأورام الدبقية. بين عامي 1934 و1941 كان الباحث الأكثر غزارة في أبحاث الأورام الدبقية هو هانز يواكيم شيرر، الذي افترض بعض الجوانب السريرية والمورفولوجية للورم الأرومي الدبقي متعدد الأشكال. مع إدخال الاختبارات الجزيئية والوراثية، تم تحديد التعدد الحقيقي لأورام الدماغ متعددة الأشكال، حيث تحمل الأنماط الجينية المختلفة نفس الصورة الهيستومورفولوجية وIHC، بالإضافة إلى بعض جوانب تكوين الورم الدبقي. لكي يتطور الورم الدماغي متعدد الأشكال، يجب أن تحدث طفرة محفزة محددة في الخلية الجذعية للورم الدماغي متعدد الأشكال - الورم الدماغي متعدد الأشكال الأولي، أو التجمع البطيء للطفرات الفردية، دون طفرة محفزة مميزة - الورم الدماغي متعدد الأشكال الثانوي. كانت معرفة الورم الدماغي متعدد الأشكال مرتبطة ارتباطًا وثيقًا بالمعرفة الطبية العامة للجهاز العصبي المركزي منذ وصف هذه الأورام الخبيثة لأول مرة منذ أكثر من 200 عام. تم تحقيق عدة قفزات كبيرة في ذلك الوقت، على خطى الجهاز العصبي المركزي والتقدم في المعرفة الطبية العامة. يتزايد الطلب على علاج الورم الدماغي متعدد الأشكال، والذي تشارك الشركات المصنعة في إطلاق المنتجات الجديدة له، وزيادة منتجات خطوط الأنابيب والمشاركة في الأحداث في السوق. تعمل هذه القرارات في نهاية المطاف على تعزيز نمو السوق.

يقدم تقرير سوق علاج الورم الأرومي الدبقي متعدد الأشكال تفاصيل عن حصة السوق والتطورات الجديدة وتأثير اللاعبين المحليين والمحليين في السوق وتحليل الفرص من حيث جيوب الإيرادات الناشئة والتغييرات في لوائح السوق وموافقات المنتجات والقرارات الاستراتيجية وإطلاق المنتجات والتوسعات الجغرافية والابتكارات التكنولوجية في السوق. لفهم التحليل وسيناريو السوق، اتصل بنا للحصول على موجز محلل، وسيساعدك فريقنا في إنشاء حل تأثير الإيرادات لتحقيق هدفك المنشود. المبادرات الاستراتيجية مثل التعاون والاتفاق وتوقيع اتفاقيات المبيعات لاختراع وابتكار علاجات دوائية هي المحركات الرئيسية التي دفعت الطلب على السوق في فترة التنبؤ.
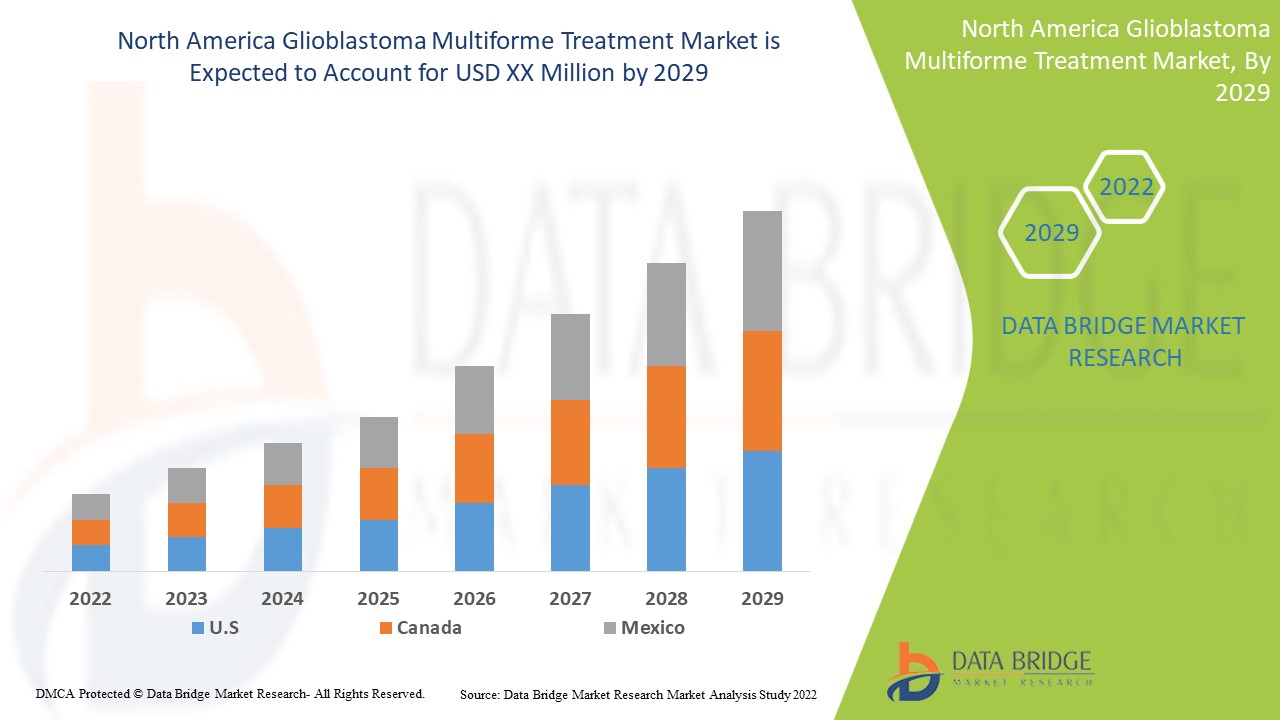
يعتبر سوق علاج الورم الأرومي الدبقي متعدد الأشكال داعمًا ويهدف إلى الحد من تطور المرض. تحلل شركة Data Bridge Market Research أن سوق علاج الورم الأرومي الدبقي متعدد الأشكال سينمو بمعدل نمو سنوي مركب بنسبة 8.5% خلال الفترة المتوقعة من 2022 إلى 2029.
|
تقرير القياس |
تفاصيل |
|
فترة التنبؤ |
2022 إلى 2029 |
|
سنة الأساس |
2021 |
|
سنوات تاريخية |
2020 (قابلة للتخصيص حتى 2019 - 2014) |
|
وحدات كمية |
الإيرادات بالملايين من الدولارات الأمريكية، التسعير بالدولار الأمريكي |
|
القطاعات المغطاة |
حسب النوع (أساسي (De Novo)، ثانوي)، العلاج (الجراحة، العلاج الإشعاعي، الأدوية)، نوع المريض (بالغ، مسن، طفل)، نوع الدواء (أدوية عامة، أدوية ذات علامة تجارية)، طريق الإعطاء (حقن، عن طريق الفم، أدوية أخرى)، المستخدم النهائي (المستشفيات، العيادات، الرعاية الصحية المنزلية، أدوية أخرى)، قناة التوزيع (صيدلية المستشفى، صيدلية التجزئة، صيدلية الإنترنت، أدوية أخرى) |
|
الدول المغطاة |
الولايات المتحدة وكندا والمكسيك |
|
الجهات الفاعلة في السوق المشمولة |
F. Hoffmann-La Roche AG، Amgen Inc.، Merck & Co.، Inc.، Pfizer Inc.، Varian Medical Systems, Inc. (شركة تابعة لشركة Siemens Healthcare)، ZEISS International، Amneal Pharmaceuticals LLC، Elekta، Sun Pharmaceutical Industries Ltd، Teva Pharmaceutical Industries Ltd.، Eckert & Ziegler، Accord Healthcare، Angiochem، ANI Pharmaceuticals, Inc.، Arbor Pharmaceuticals, LLC. (شركة تابعة لشركة Azurity Pharmaceuticals, Inc.)، وAstraZeneca، وCantex Pharmaceuticals, Inc.، وCELON LABS، وDiffusion Pharmaceuticals Inc.، وEnGeneIC، وERC.SA.، وGenenta science، وJazz Pharmaceuticals, Inc.، وLoxo Oncology (شركة تابعة لشركة Eli Lilly)، وNovartis AG، وVBL THERAPEUTICS، وViatris Inc.، وZydus Pharmaceuticals, Inc.، وغيرها. |
تعريف السوق
الورم الأرومي الدبقي متعدد الأشكال هو الورم الخبيث الأولي الأكثر شيوعًا وعدوانية في المخ ويمثل 60% من أورام المخ لدى البالغين. يمكن أن ينشأ الورم الأرومي الدبقي متعدد الأشكال في المخ حديثًا أو يتطور من ورم نجمي منخفض الدرجة. في البالغين، يحدث الورم الأرومي الدبقي متعدد الأشكال غالبًا في نصفي الكرة المخية، وخاصة في الفص الجبهي والصدغي من المخ. تمت دراسة العديد من العوامل الوراثية والبيئية في الورم الأرومي الدبقي متعدد الأشكال، ولكن لم يتم تحديد عامل خطر مسؤول عن نسبة كبيرة من الورم الأرومي الدبقي متعدد الأشكال. لذا، مثل العديد من أنواع السرطان الأخرى، يكون الورم الأرومي الدبقي متعدد الأشكال متقطعًا، على الرغم من أن بعض الدراسات تشير إلى انتشار مرتفع (17%) للإشعاع العلاجي السابق بين المرضى المصابين بالورم الأرومي الدبقي متعدد الأشكال. يختلف الكمون بين الإشعاع وتطور الورم الأرومي الدبقي متعدد الأشكال من بضع سنوات إلى عدة عقود. لا يوجد دليل ملموس على ارتباط الورم الأرومي الدبقي متعدد الأشكال بعوامل نمط الحياة مثل التدخين أو استهلاك الكحول أو تعاطي المخدرات أو التعرض لمركبات N- Nitroso. وأظهرت الدراسات أن استخدام الهواتف المحمولة لا يزيد من خطر الإصابة بالورم الدبقي متعدد الأشكال؛ ومع ذلك، فإن ارتباطه بالاستخدام طويل الأمد يحتاج إلى مزيد من التأكيد.
ديناميكيات سوق علاج الورم الأرومي الدبقي متعدد الأشكال
السائقين
- انتشار متزايد لمرض الورم الأرومي الدبقي متعدد الأشكال
الورم الأرومي الدبقي متعدد الأشكال (GBM) هو الورم الدماغي الأولي الخبيث الأكثر شيوعًا، ويمثل 77٪ -81٪ من جميع الأورام الخبيثة الأولية في الجهاز العصبي المركزي (CNS). صنفته منظمة الصحة العالمية على أنه ورم نجمي منتشر من الدرجة الرابعة وقليل التغصنات. متوسط عمر ظهور الورم الأرومي الدبقي متعدد الأشكال الأولي هو 62 عامًا، ومتوسط البقاء على قيد الحياة حوالي 14.6 شهرًا. تم توثيق التشخيص السيئ المرتبط بالورم الأرومي الدبقي متعدد الأشكال جيدًا، في حين تظل معدلات البقاء على قيد الحياة منخفضة بشكل مخيب للآمال على الرغم من التقدم الطبي والجراحي. وفقًا للدراسة، تكشف الدراسات الدولية عن معدل حدوث سنوي تقريبي يتراوح من 0.59 إلى 5 لكل 100000 شخص؛ ومع ذلك، تشير الدراسات إلى ارتفاع في الإصابة. وصف ميراندا فيلهو وآخرون في عام 2017 معدلات متزايدة لسرطانات الجهاز العصبي المركزي والدماغ في دول في أمريكا الجنوبية وأوروبا الشرقية وجنوب أوروبا، بينما تم الإبلاغ عن انخفاض المعدلات في اليابان فقط. دوبس وآخرون. في عام 2011، لاحظ الباحثون أيضًا زيادة في حالات أورام GBM في دراستين أستراليتين متعددتي المراكز، مع زيادة خاصة في أورام GBM في الفص الجبهي والصدغي. إن زيادة حالات الإصابة بأورام الدماغ المتعددة الأشكال تزيد من الطلب على الكشف المبكر والتشخيص من خلال الاستفادة من أحدث التقنيات، مما يدفع سوق علاج أورام الدماغ المتعددة الأشكال العالمية إلى الأمام. ومن المتوقع أن يؤدي ارتفاع حالات الإصابة بأورام الدماغ المتعددة الأشكال في جميع أنحاء العالم إلى تسريع الطلب على علاج أورام الدماغ المتعددة الأشكال. وبالتالي، من المتوقع أن تعزز معدلات الإصابة المتزايدة بأورام الدماغ المتعددة الأشكال نمو السوق.
- زيادة البحث والتطوير
لقد سهّلت أنشطة البحث والتطوير المتزايدة في مجال التكنولوجيا الحيوية الجزيئية والعلاج الجيني للسرطان والأمراض ذات الصلة تطوير العديد من الأدوية البيولوجية. تساعد هذه الأدوية في تقليل الآثار الجانبية لطرق العلاج الحالية، وبالتالي خلق قبول أوسع بين المرضى. ومن المتوقع أن يؤدي تباين الأورام والاختلاف في نهج العلاج من مريض إلى آخر إلى زيادة الطلب على نهج علاجي شخصي لإدارة الورم الأرومي الدبقي متعدد الأشكال. ومن المتوقع أن يؤدي اعتماد علاجات جديدة إلى زيادة متوسط العمر المتوقع للمرضى الذين يعيشون مع الورم الأرومي الدبقي متعدد الأشكال. وعلاوة على ذلك، من المتوقع أن يؤدي التصنيف الخاص الممنوح للأدوية التجريبية من قبل إدارة الغذاء والدواء إلى تسريع عملية الموافقة على العلاج الجديد والتسويق. ومن المتوقع أن يؤدي زيادة التعاون بين الباحثين واللاعبين في السوق إلى تعزيز تطوير خيارات علاجية جديدة وفعالة للورم الأرومي الدبقي متعدد الأشكال. ومن المتوقع أن يؤدي الموافقة المتزايدة على العلاج الجديد والعلاج المركب إلى دفع سوق علاج الورم الأرومي الدبقي متعدد الأشكال.
فرصة
- زيادة الموافقات على الأدوية
إن الطلب المتزايد على علاج الورم الأرومي الدبقي متعدد الأشكال من شأنه أن يؤدي إلى الحصول على المزيد من الموافقات التنظيمية للأدوية المرتبطة به. وسوف تشكل الموافقات التنظيمية المتزايدة للأدوية المرتبطة به والمنتجات المعاد تركيبها زيادة في قيمة سوق علاج الورم الأرومي الدبقي متعدد الأشكال في السنوات القادمة. وفي إطار مبادرة منظمة الصحة للبلدان الأمريكية (PAHO) لتعزيز الاعتراف بسلطات تنظيم الأدوية، انتهت عملية تقييم ANMAT في 11 ديسمبر 2009. وقد شهدت صناعة علاج الورم الأرومي الدبقي متعدد الأشكال العديد من الموافقات على الأدوية في السنوات الأخيرة، مدفوعة بمعدل الوفيات المتزايد بسبب هذا المرض. وسوف تعمل الموافقات المتزايدة على زيادة الطلب على سوق علاج الورم الأرومي الدبقي متعدد الأشكال.
القيود/التحديات
التكلفة العالية لعلاج ورم أرومي دبقي متعدد الأشكال
تتضمن الاختبارات التشخيصية لورم الدماغ المتعدد الأشكال منتجات متقدمة تقنيًا للغاية. يتطلب تطوير هذه المنتجات بحثًا وتطويرًا صارمين من قبل الشركة المطورة. وبالتالي، تظل تكلفة المنتج مرتفعة، مما يزيد بشكل متناسب من تكلفة الاختبار.
تشمل الأدوات والتقنيات التشخيصية المستخدمة لتشخيص الورم الأرومي الدبقي متعدد الأشكال ما يلي:
العلاج الإشعاعي والعلاج الكيميائي، من بين أمور أخرى. تظهر المراحل المبكرة من الورم الأرومي الدبقي متعدد الأشكال عادةً بأعراض بسيطة أو بدون أعراض على الإطلاق؛ لذلك، غالبًا ما يتم تشخيص الورم الأرومي الدبقي متعدد الأشكال في مراحل متقدمة، مما يؤدي إلى تشخيص سيئ. وبالتالي، فإن التكلفة العالية لعلاج الورم الأرومي الدبقي متعدد الأشكال باستخدام الوسائل والمنتجات التكنولوجية المتقدمة ستعمل كعامل تقييد رئيسي لنمو سوق علاج الورم الأرومي الدبقي متعدد الأشكال العالمي.
التطورات الأخيرة
- في أبريل 2022، أعلنت شركة إليكتا وجنرال إلكتريك للرعاية الصحية عن توقيع اتفاقية تعاون تجاري عالمي في مجال علاج الأورام بالإشعاع، مما يمكنهم من تقديم عرض شامل للمستشفيات عبر التصوير والعلاج لمرضى السرطان الذين يحتاجون إلى العلاج الإشعاعي. ستسمح هذه الشراكة للشركتين بالترويج المشترك للحلول لتلبية احتياجات كل مركز للسرطان
- في يوليو 2019، أعلنت شركة أمجين وشركة أليرجان أن عقار MVASI (bevacizumab-awwb)، وهو عقار مشابه حيويًا لعقار أفاستين (bevacizumab)، متاح في الولايات المتحدة. ومن شأن هذا الإطلاق أن يعزز مبيعات المنتج في المنطقة
نطاق سوق علاج الورم الأرومي الدبقي متعدد الأشكال
يتم تصنيف سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى سبعة قطاعات بارزة تعتمد على النوع والعلاج ونوع المريض ونوع الدواء وطريقة الإدارة والمستخدم النهائي وقناة التوزيع. يساعدك النمو بين القطاعات على تحليل جيوب النمو والاستراتيجيات المتخصصة للتعامل مع السوق وتحديد مجالات التطبيق الأساسية والاختلاف في الأسواق المستهدفة.
يكتب
- الابتدائية (دي نوفو)
- ثانوي
على أساس النوع، يتم تقسيم سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى أولي (De Novo) وثانوي.
علاج
- جراحة
- العلاج الإشعاعي
- الأدوية
على أساس العلاج، يتم تقسيم سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى الجراحة والعلاج الإشعاعي والأدوية.
نوع المريض
- بالغ
- كبار السن
- طفل
على أساس نوع المريض، يتم تقسيم سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى البالغين وكبار السن والأطفال.
نوع الدواء
- العلامة التجارية
- الأنواع العامة
على أساس نوع الدواء، يتم تقسيم سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى أدوية عامة وأدوية ذات علامات تجارية.
طريق الإدارة
- شفوي
- الحقن الوريدي
- آحرون
على أساس طريق الإدارة، يتم تقسيم سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى علاج بالحقن والفم وغيرها.
المستخدم النهائي
- المستشفيات
- العيادات
- الرعاية الصحية المنزلية
- آحرون
على أساس المستخدم النهائي، يتم تقسيم سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى المستشفيات والعيادات والرعاية الصحية المنزلية وغيرها.
قناة التوزيع
- صيدلية المستشفى
- صيدلية التجزئة
- صيدلية على الإنترنت
- آحرون
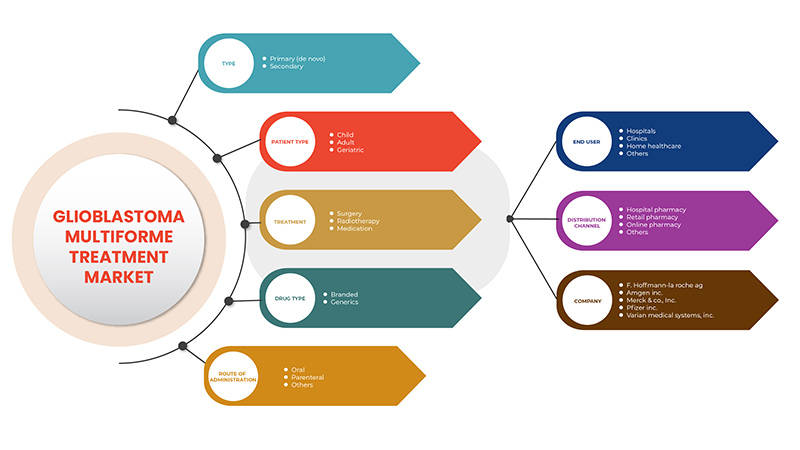
على أساس قناة التوزيع، يتم تقسيم سوق علاج الورم الأرومي الدبقي متعدد الأشكال إلى صيدلية المستشفيات وصيدلية التجزئة وغيرها.
تحليل إقليمي/رؤى حول سوق علاج الورم الأرومي الدبقي متعدد الأشكال العالمي
يتم تحليل سوق علاج الورم الأرومي الدبقي متعدد الأشكال ويتم توفير رؤى حجم السوق والاتجاهات حسب النوع والعلاج ونوع المريض ونوع الدواء وطريقة الإدارة والمستخدم النهائي وقناة التوزيع كما هو مذكور أعلاه.
المناطق التي يغطيها تقرير سوق علاج الورم الأرومي الدبقي متعدد الأشكال هي الولايات المتحدة وكندا والمكسيك.
وفي أمريكا الشمالية، من المتوقع أن تهيمن الولايات المتحدة على السوق بسبب الانتشار المتزايد للمرض في المنطقة.
كما يوفر قسم الدولة في التقرير عوامل التأثير الفردية على السوق والتغيرات في تنظيم السوق التي تؤثر على الاتجاهات الحالية والمستقبلية للسوق. نقاط البيانات مثل تحليل سلسلة القيمة المصب والمصب، والاتجاهات الفنية وتحليل قوى بورتر الخمس، ودراسات الحالة هي بعض المؤشرات المستخدمة للتنبؤ بسيناريو السوق للدول الفردية. كما يتم النظر في وجود وتوافر العلامات التجارية العالمية والتحديات التي تواجهها بسبب المنافسة الكبيرة أو النادرة من العلامات التجارية المحلية والمحلية، وتأثير التعريفات الجمركية المحلية وطرق التجارة أثناء تقديم تحليل توقعات لبيانات الدولة.
تحليل المنافسة وحصة سوق علاج الورم الأرومي الدبقي متعدد الأشكال
يوفر المشهد التنافسي لسوق علاج الورم الأرومي الدبقي متعدد الأشكال تفاصيل عن المنافسين. تتضمن التفاصيل نظرة عامة على الشركة، والبيانات المالية للشركة، والإيرادات المتولدة، وإمكانات السوق، والاستثمار في البحث والتطوير، ومبادرات السوق الجديدة، والحضور العالمي، ومواقع الإنتاج والمرافق، والقدرات الإنتاجية، ونقاط القوة والضعف في الشركة، وإطلاق المنتج، وعرض المنتج ونطاقه، وهيمنة التطبيق. ترتبط نقاط البيانات المذكورة أعلاه فقط بتركيز الشركات على سوق علاج الورم الأرومي الدبقي متعدد الأشكال.
بعض اللاعبين الرئيسيين في السوق هم F. Hoffmann-La Roche AG، Amgen Inc.، Merck & Co.، Inc.، Pfizer Inc.، Varian Medical Systems، Inc. (شركة تابعة لشركة Siemens Healthcare)، ZEISS International، Amneal Pharmaceuticals LLC، Elekta، Sun Pharmaceutical Industries Ltd، Teva Pharmaceutical Industries Ltd.، Eckert & Ziegler، Accord Healthcare، Angiochem، ANI Pharmaceuticals، Inc.، Arbor Pharmaceuticals، LLC. (شركة تابعة لشركة Azurity Pharmaceuticals, Inc.)، وAstraZeneca، وCantex Pharmaceuticals, Inc.، وCELON LABS، وDiffusion Pharmaceuticals Inc.، وEnGeneIC، وERC.SA.، وGenenta science، وJazz Pharmaceuticals, Inc.، وLoxo Oncology (شركة تابعة لشركة Eli Lilly)، وNovartis AG، وVBL THERAPEUTICS، وViatris Inc.، وZydus Pharmaceuticals, Inc.، وغيرها.
منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. بالإضافة إلى ذلك، يعد تحليل حصة السوق وتحليل الاتجاهات الرئيسية من عوامل النجاح الرئيسية في تقرير السوق. منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي مثلث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأساسي (خبير الصناعة). وبصرف النظر عن هذا، تتضمن نماذج البيانات شبكة وضع البائعين وتحليل الخط الزمني للسوق ونظرة عامة على السوق والدليل وشبكة وضع الشركة وتحليل حصة الشركة في السوق ومعايير القياس والتحليل العالمي مقابل الإقليمي وتحليل حصة البائعين. يرجى طلب مكالمة محلل في حالة وجود استفسار آخر.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS
7 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: REGULATORY SCENARIO
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROWING PREVALENCE OF GLIOBLASTOMA MULTIFORME
8.1.2 INCREASING RESEARCH AND DEVELOPMENT (R&D)
8.1.3 PRESENCE OF A STRONG PIPELINE
8.1.4 GROWING GERIATRIC POPULATION
8.2 RESTRAINTS
8.2.1 HIGH COST OF GLIOBLASTOMA MULTIFORME TREATMENT
8.2.2 ADVERSE SIDE-EFFECTS OF GLIOBLASTOMA MULTIFORME TREATMENT
8.3 OPPORTUNITIES
8.3.1 INCREASING DRUG APPROVALS
8.3.2 PARTNERSHIP AND AGREEMENT BY MAJOR PLAYERS
8.3.3 INCREASING SUPPORT OF PRIVATE AND GOVERNMENT AGENCIES FOR TREATMENT
8.4 CHALLENGES
8.4.1 LACK OF NEW TREATMENT
8.4.2 ADVERSE EFFECTS AND RISKS ASSOCIATED WITH CANCER TREATMENT DRUGS
8.4.3 LACK OF EARLY DETECTION
9 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE
9.1 OVERVIEW
9.2 PRIMARY (DE NOVO)
9.3 SECONDARY
10 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT
10.1 OVERVIEW
10.2 SURGERY
10.3 RADIOTHERAPY
10.3.1 BRACHYTHERAPY
10.3.2 FRACTIONATED STEREOTACTIC RT (FSRT)
10.3.3 CONFORMAL OR INTENSITY-MODULATED RT
10.3.4 RADIOSURGERY
10.4 MEDICATIONS
10.4.1 TEMOZOLOMIDE
10.4.1.1 ORAL
10.4.1.1 INTRAVENOUS
10.4.2 NITROSOUREAS DRUGS
10.4.2.1 CARMUSTINE
10.4.2.1.1 PARENTERAL
10.4.2.1.2 IMPLANTABLE WAFERS
10.4.2.2 LOMUSTINE
10.4.2.3 NIMUSTINE
10.4.2.4 FOTEMUSTINE
10.4.3 TARGETED THERAPY
10.4.3.1 BEVACIZUMAB
10.4.3.2 OTHERS
10.4.4 ANTI-EPILEPTICS
10.4.4.1 LEVETIRACETAM
10.4.4.2 PHENYTOIN
10.4.4.3 CARBAMAZEPINE
10.4.5 CORTICOSTEROIDS
10.4.5.1 METHYLPREDNISOLONE
10.4.5.2 PREDNISONE
10.4.5.3 OTHERS
11 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE
11.1 OVERVIEW
11.2 ADULT
11.2.1 MALE
11.2.2 FEMALE
11.3 GERIATRIC
11.3.1 MALE
11.3.2 FEMALE
11.4 CHILD
12 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERICS
12.3 BRANDED
13 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 PARENTERAL
13.3 ORAL
13.3.1 CAPSULES
13.3.2 TABLETS
13.3.3 POWDERS
13.4 OTHERS
14 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITAL
14.3 CLINICS
14.4 HOME HEALTHCARE
14.5 OTHERS
15 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 HOSPITAL PHARMACY
15.3 RETAIL PHARMACY
15.4 ONLINE PHARMACY
15.5 OTHERS
16 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 F.HOFFMAN-LA ROCHE
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 AMGEN INC.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.2.5.1 PRODUCT APPROVAL
19.3 MERCK & CO., INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.3.5.1 STRATETIC COLLABORATION
19.3.5.2 EVENTS
19.4 PFIZER INC.
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.4.5.1 MERGER
19.5 VARIAN MEDICAL SYSTEMS, INC. (A SUBSIDIARY OF SIEMENS HEALTHCARE)
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.5.5.1 PARTNERSHIP
19.5.5.2 ACQUISITION
19.6 ZEISS INTERNATIONAL
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.6.4.1 PRODUCT EXPANSION
19.7 AMNEAL PHARMACEUTICALS LLC
19.7.1 COMPANY SNAPSHOT
19.7.2 REVENUE ANALYSIS
19.7.3 PRODUCT PORTFOLIO
19.7.4 RECENT DEVELOPMENTS
19.7.4.1 EVENT
19.7.4.2 LAUNCH
19.7.4.3 ACQUISITION
19.8 ELEKTA
19.8.1 COMPANY SNAPSHOT
19.8.2 REVENUE ANALYSIS
19.8.3 PRODUCT PORTFOLIO
19.8.4 RECENT DEVELOPMENTS
19.8.4.1 PARTNERSHIP
19.9 SUN PHARMACEUTICAL INDUSTRIES LTD
19.9.1 COMPANY SNAPSHOT
19.9.2 REVENUE ANALYSIS
19.9.3 PRODUCT PORTFOLIO
19.9.4 RECENT DEVELOPMENT
19.9.4.1 AGREEMENT
19.1 TEVA PHARMACEUTICAL INDUSTRIES LTD
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 ECKERT & ZIEGLER
19.11.1 COMPANY SNAPSHOT
19.11.2 REVENUE ANALYSIS
19.11.3 PRODUCT PORTFOLIO
19.11.4 RECENT DEVELOPMENT
19.12 ACCORD HEALTHCARE
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 ANGIOCHEM
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENT
19.13.3.1 AGREMEENT
19.14 ANI PHARMACEUTICALS, INC.
19.14.1 COMPANY SNAPSHOT
19.14.2 REVENUE ANALYSIS
19.14.3 PRODUCT PORTFOLIO
19.14.4 RECENT DEVELOPMENTS
19.14.4.1 ACQUISITION
19.15 ARBOR PHARMACEUTICALS, LLC. A SUBSIDIARY OF AZURITY PHARMACEUTICALS, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENT
19.15.3.1 ACQUISITION
19.15.3.2 PRODUCT APPROVAL
19.16 ASTRAZENECA
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.16.4.1 AGREEMENT
19.17 CANTEX PHARMACEUTICALS, INC.
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENT
19.18 CELON LABS
19.18.1 COMPANY SNAPSHOT
19.18.2 PRODUCT PORTFOLIO
19.18.3 RECENT DEVELOPMENT
19.19 DIFFUSION PHARMACEUTICAL
19.19.1 COMPANY SNAPSHOT
19.19.2 SERVICES PORTFOLIO
19.19.3 RECENT DEVELOPMENT
19.2 ERC.SA
19.20.1 COMPANY SNAPSHOT
19.20.2 PRODUCT PORTFOLIO
19.20.3 RECENT DEVELOPMENT
19.20.3.1 PIPELINE UPDATE
19.21 ENGENEIC
19.21.1 COMPANY SNAPSHOT
19.21.2 PRODUCT PORTFOLIO
19.21.3 RECENT DEVELOPMENTS
19.21.3.1 AWARDS
19.22 GENENTA SCIENCE
19.22.1 COMPANY SNAPSHOT
19.22.2 PRODUCT PORTFOLIO
19.22.3 RECENT DEVELOPMENT
19.22.3.1 EVENT
19.23 JAZZ PHARMACEUTICALS, INC.
19.23.1 COMPANY SNAPSHOT
19.23.2 REVENUE ANALYSIS
19.23.3 PRODUCT PORTFOLIO
19.23.4 RECENT DEVELOPMENT
19.23.4.1 ACQUISITION
19.24 LOXO ONCOLOGY (A SUBSIDIARY OF ELI LILLY)
19.24.1 COMPANY SNAPSHOT
19.24.2 PRODUCT PORTFOLIO
19.24.3 RECENT DEVELOPMENT
19.25 NOVARTIS AG
19.25.1 COMPANY SNAPSHOT
19.25.2 REVENUE ANALYSIS
19.25.3 PRODUCT PORTFOLIO
19.25.4 RECENT DEVELOPMENT
19.26 VBL THERAPEUTICS
19.26.1 COMPANY SNAPSHOT
19.26.2 PRODUCT PORTFOLIO
19.26.3 RECENT DEVELOPMENT
19.26.3.1 EVENT
19.26.3.2 AWARD
19.27 VIATRIS INC
19.27.1 COMPANY SNAPSHOT
19.27.2 REVENUE ANALYSIS
19.27.3 PRODUCT PORTFOLIO
19.27.4 RECENT DEVELOPMENT
19.27.4.1 AGREEMENT
19.28 ZYDUS PHARMACEUTICALS, INC.
19.28.1 COMPANY SNAPSHOT
19.28.2 PRODUCT PORTFOLIO
19.28.3 RECENT DEVELOPMENTS
20 QUESTIONNAIRE
21 RELATED REPORTS
List of Table
TABLE 1 PIPELINE ANALYSIS FOR GLIOBLASTOMA MULTIFORME TREATMENT MARKET
TABLE 2 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA PRIMARY (DE NOVO) IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA SECONDARY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA SURGERY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA ADULTS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CHILD IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA GENERICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA BRANDED IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA PARENTERAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA HOSPITAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA CLINICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA HOME HEALTHCARE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA HOSPITAL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA RETAIL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA ONLINE PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 56 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 57 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 58 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 59 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 60 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 61 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 62 U.S. RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 63 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 64 U.S. TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 65 U.S. NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 66 U.S. CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 67 U.S. TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 68 U.S. ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 69 U.S. CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 70 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 71 U.S. ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 72 U.S. GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 73 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 74 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 75 U.S. ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 76 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 77 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 78 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 80 CANADA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 81 CANADA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 82 CANADA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 83 CANADA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 84 CANADA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 85 CANADA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 86 CANADA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 87 CANADA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 88 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 89 CANADA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 90 CANADA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 91 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 92 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 93 CANADA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 94 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 96 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 97 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 98 MEXICO RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 99 MEXICO MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 100 MEXICO TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 101 MEXICO NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 102 MEXICO CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 103 MEXICO TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 104 MEXICO ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 105 MEXICO CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 106 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 107 MEXICO ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 108 MEXICO GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 109 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 110 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 111 MEXICO ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 112 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET : DATA TRIANGULATION
FIGURE 3 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MULTIVARIATE MODELLING
FIGURE 7 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 12 NORTH AMERICA IS EXPECTED TO DOMINATE AND ASIA-PACIFIC IS GROWING AT THE FASTEST PACE IN NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 13 INCREASE IN THE PREVALENCE OF GLIOBLASTOMA MULTIFORME AND INCREASE IN PIPELINE PRODUCTS ARE DRIVING THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 PRIMARY (DE NOVO) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN 2022 & 2029
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
FIGURE 16 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2021
FIGURE 17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 18 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 19 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2021
FIGURE 21 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2022-2029 (USD MILLION)
FIGURE 22 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, CAGR (2022-2029)
FIGURE 23 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, LIFELINE CURVE
FIGURE 24 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2021
FIGURE 25 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 26 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 27 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 28 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2021
FIGURE 29 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2022-2029 (USD MILLION)
FIGURE 30 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, CAGR (2022-2029)
FIGURE 31 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 32 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 33 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD MILLION)
FIGURE 34 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 35 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 36 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2021
FIGURE 37 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 38 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, CAGR (2022-2029)
FIGURE 39 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 41 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 42 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 43 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SNAPSHOT (2021)
FIGURE 45 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021)
FIGURE 46 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2022 & 2029)
FIGURE 47 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021 & 2029)
FIGURE 48 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE (2022-2029)
FIGURE 49 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY SHARE 2021 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.