السوق العالمية لعلاج ضمور العضلات دوشين، حسب نوع العلاج (العلاجات القائمة على الجزيئات، والعلاج بالستيرويد، وغيرها)، والعلاج (نهج تخطي الإكسون، وقمع الطفرات، والعلاجات المستهدفة للديستروفين)، وطريق الإدارة (عن طريق الفم، والحقن، وغيرها)، والمستخدم النهائي (المستشفيات، والعيادات المتخصصة، والرعاية المنزلية، وغيرها)، وقناة التوزيع (صيدليات المستشفيات، والصيدليات بالتجزئة، والصيدليات عبر الإنترنت) - اتجاهات الصناعة وتوقعاتها حتى عام 2030.
تحليل حجم سوق علاج ضمور العضلات دوشين
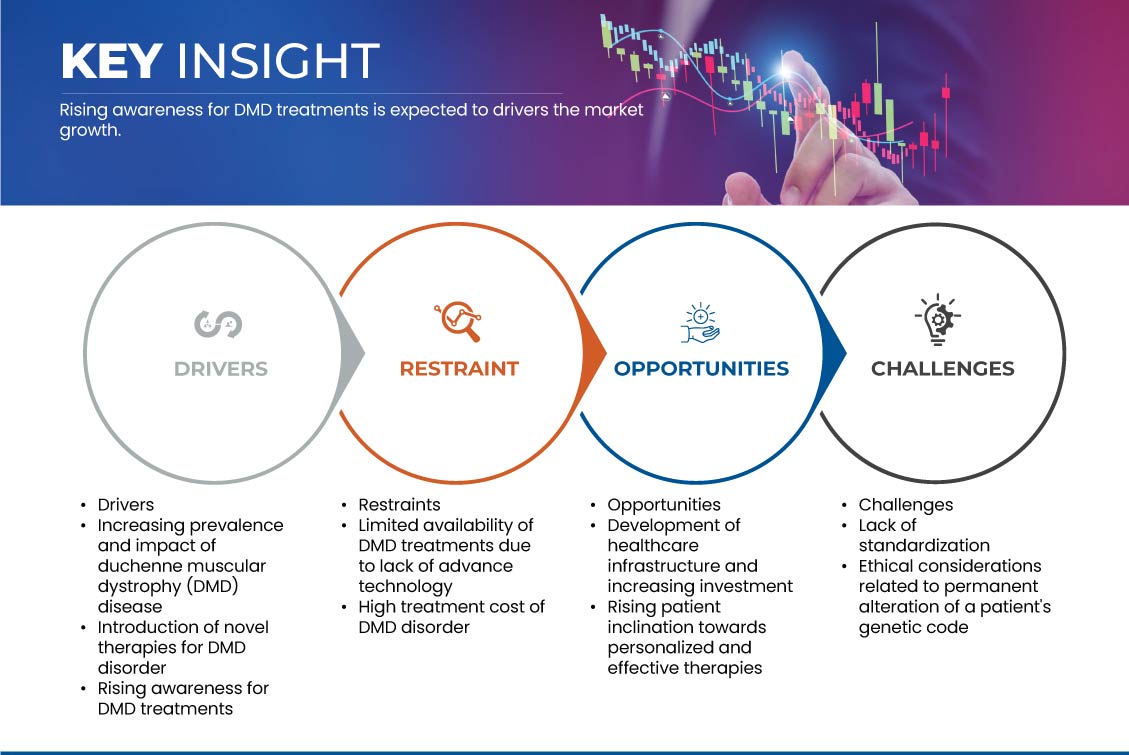
العوامل الأساسية المتوقعة لدفع نمو السوق هي الوعي المتزايد بعلاج دوشين وإدخال علاجات جديدة لاضطراب دوشين. بالإضافة إلى ذلك، فإن ارتفاع معدل انتشار وتأثير اضطراب دوشين هو محرك رئيسي آخر من المتوقع أن يدفع نمو السوق. ومن المتوقع أيضًا أن يؤدي ارتفاع عدد التجارب السريرية وهو اتجاه حديث إلى دفع نمو السوق. ومع ذلك، من المتوقع أن يؤدي التوافر المحدود لعلاج دوشين بسبب التكنولوجيا المتقدمة والتكلفة العالية لعلاج اضطراب دوشين إلى كبح نمو السوق.
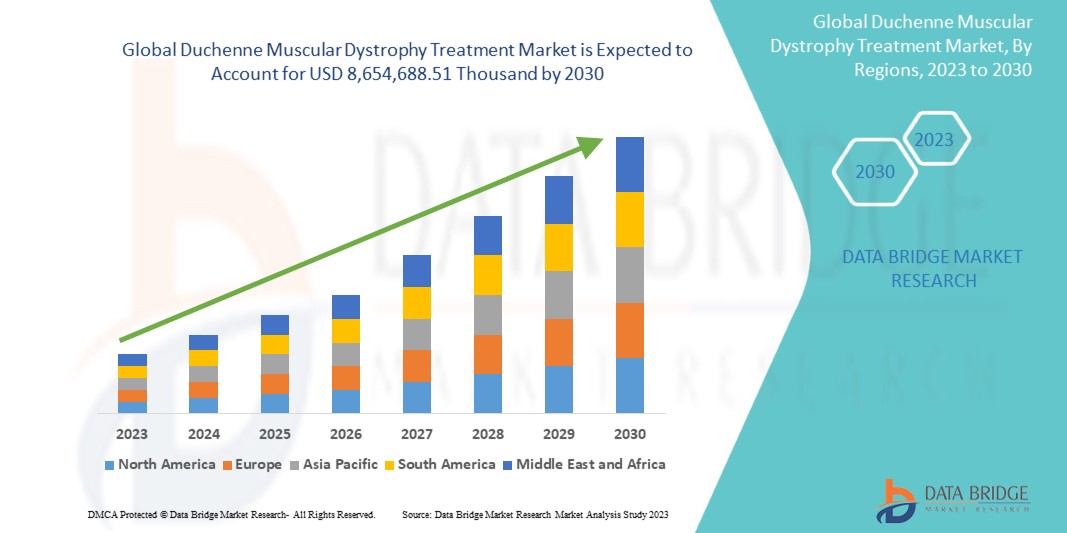
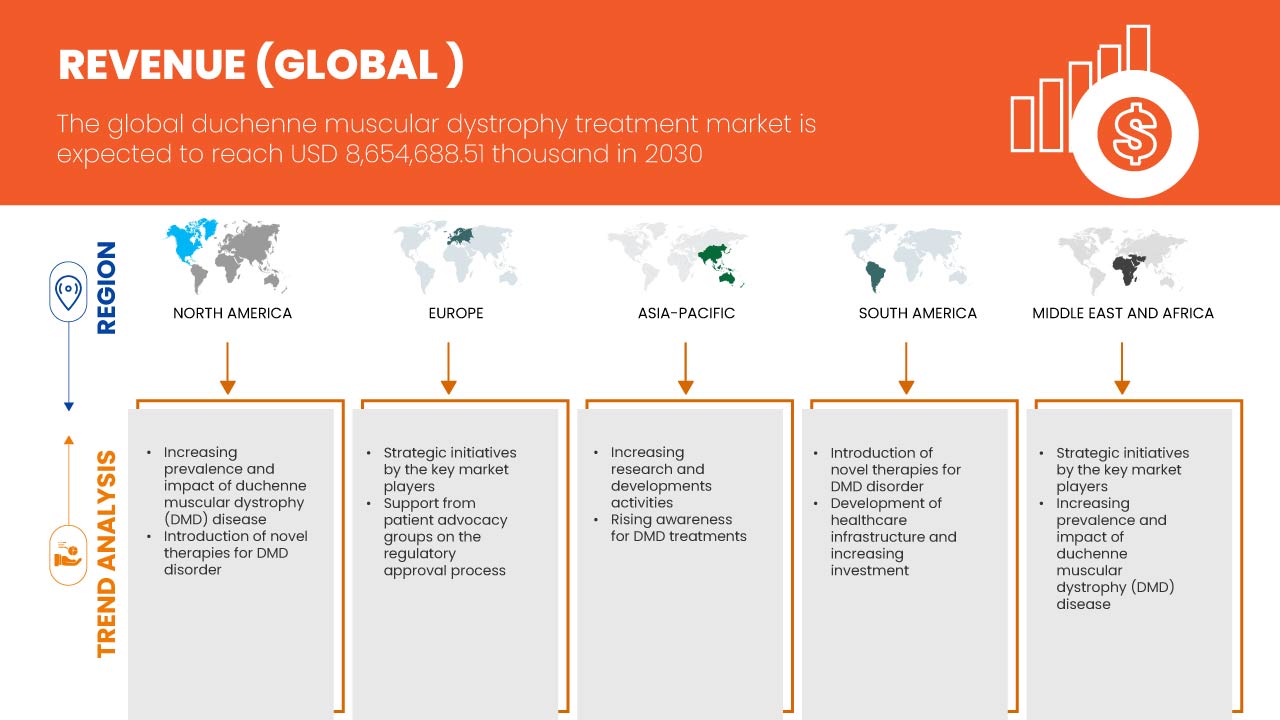
تحلل شركة Data Bridge Market Research أن سوق علاج ضمور العضلات دوشين العالمي من المتوقع أن يصل إلى 8,654,688.51 ألف دولار أمريكي بحلول عام 2030، بمعدل نمو سنوي مركب قدره 16.8% خلال الفترة المتوقعة 2023-2030. يغطي تقرير السوق هذا أيضًا تحليل الأسعار والتقدم التكنولوجي بعمق.
|
تقرير القياس |
تفاصيل |
|
فترة التنبؤ |
2023 إلى 2030 |
|
سنة الأساس |
2022 |
|
سنوات تاريخية |
2021 (قابلة للتخصيص حتى 2015 - 2020) |
|
وحدات كمية |
الإيرادات بالألف دولار أمريكي |
|
القطاعات المغطاة |
نوع العلاج (العلاجات القائمة على الجزيئات، والعلاج بالستيرويد، وغيرها)، العلاج (نهج تخطي الإكسون، وقمع الطفرات، والعلاجات المستهدفة للديستروفين)، طريق الإدارة (عن طريق الفم، والحقن، وغيرها)، المستخدم النهائي (المستشفيات، والعيادات المتخصصة، والرعاية المنزلية، وغيرها)، قناة التوزيع (صيدلية المستشفى، وصيدلية التجزئة، والصيدلية عبر الإنترنت) |
|
الدول المغطاة |
الولايات المتحدة، كندا، المكسيك، ألمانيا، فرنسا، المملكة المتحدة، إيطاليا، إسبانيا، روسيا، تركيا، بلجيكا، هولندا، سويسرا، بقية أوروبا، اليابان، الصين، كوريا الجنوبية، الهند، أستراليا، سنغافورة، تايلاند، ماليزيا، إندونيسيا، الفلبين، بقية دول آسيا والمحيط الهادئ، البرازيل، الأرجنتين، بقية دول أمريكا الجنوبية، جنوب أفريقيا، المملكة العربية السعودية، الإمارات العربية المتحدة، مصر، إسرائيل، وبقية دول الشرق الأوسط وأفريقيا |
|
الجهات الفاعلة في السوق المشمولة |
Sarepta Therapeutics, Inc.، وGSK plc.، وCapricor Therapeutics, Inc.، وDyne Therapeutics، وSolid Biosciences Inc.، وBioMarin، وStealth BioTherapeutics Inc.، وAvidity Biosciences، وReveraGen BioPharma, Inc.، وPTC Therapeutics.، وNS Pharma, Inc، وITALFARMACO SpA، وFibroGen, Inc، وSANTHERA PHARMACEUTICALS، وPfizer Inc.، وF. Hoffmann-La Roche Ltd، وAkashi RX، وTAIHO PHARMACEUTICAL CO., LTD وغيرها |
تعريف السوق
ضمور العضلات دوشين (DMD) هو اضطراب وراثي نادر يتميز بتدهور العضلات التدريجي والضعف. يتضمن العلاج الأساسي لضمور العضلات دوشين استخدام الكورتيكوستيرويدات، مثل بريدنيزون أو ديفلازاكورت. تساعد هذه الأدوية في تقليل الالتهاب وتأخير تدهور العضلات، مما يؤدي في النهاية إلى إطالة القدرة على المشي والحفاظ على وظيفة العضلات. وقد ثبت أن علاج الكورتيكوستيرويد يحسن قوة العضلات ووظيفة الجهاز التنفسي وجودة الحياة بشكل عام لدى الأفراد المصابين بضمور العضلات دوشين.
ديناميكيات السوق العالمية لعلاج ضمور العضلات دوشين
يتناول هذا القسم فهم محركات السوق والمزايا والقيود والتحديات. وسيتم مناقشة كل هذا بالتفصيل أدناه:
السائقين
- تزايد انتشار وتأثير مرض ضمور العضلات دوشين (DMD)
ضمور العضلات دوشين (DMD) هو اضطراب وراثي نادر وموهن يتميز بضعف العضلات التدريجي وفقدان الوظيفة. ويؤثر بشكل أساسي على الذكور، وتظهر الأعراض عادة في مرحلة الطفولة المبكرة. كما يتزايد عدد الأفراد الذين تم تشخيصهم بضمور العضلات دوشين، وقد أدى هذا الانتشار المتزايد إلى خلق الحاجة إلى علاجات وأساليب علاج فعالة، مع استمرار نمو عدد السكان في جميع أنحاء العالم.
كما أدى الانتشار المتزايد لمرض دوشين إلى زيادة جهود المناصرة من قبل المرضى ومقدمي الرعاية الصحية. تلعب هذه المجموعات دورًا حيويًا في زيادة الوعي بمرض دوشين، والدعوة إلى تمويل الأبحاث، والموافقة بشكل أسرع على العلاجات المحتملة. يتزايد مجتمع دوشين، وبالتالي، فإن تقدم التكنولوجيات والاستثمارات في هذا المجال من الرعاية الصحية أمر مهم أيضًا.
- تقديم علاجات جديدة لاضطراب دوشين العضلي
يؤدي ظهور أساليب العلاج المبتكرة إلى زيادة رعاية مرضى دوشين والمساهمة بشكل كبير في توسيع السوق. تركز خيارات العلاج التقليدية في المقام الأول على إدارة الأعراض والرعاية الداعمة. ومع ذلك، فإن إدخال علاجات جديدة يمثل تحولاً جذريًا لأنها تهدف إلى معالجة الطفرات الجينية الجذرية المسؤولة عن دوشين.
وعلاوة على ذلك، ظهرت أدوية تخطي الإكسونات كفئة واعدة أخرى من العلاجات لمرض دوشين. وقد صُممت هذه الأدوية لتخطي إكسونات محددة في جين الديستروفين، مما يسمح بإنتاج بروتين ديستروفين مقطوع ولكنه يعمل جزئيًا. ويمكن لهذه الأدوية أن تبطئ بشكل كبير من تقدم المرض وتحسن نوعية الحياة للأفراد المصابين بمرض دوشين. وقد أدى تطوير هذه العلاجات والموافقة عليها إلى تجديد التوقعات لدى مرضى دوشين، ومن المتوقع أن يؤدي ذلك إلى دفع نمو السوق.
فرصة
- تطوير البنية التحتية للرعاية الصحية وزيادة الاستثمار
يمكن تشخيص المرضى المصابين بمرض دوشين في وقت مبكر من خلال بنية تحتية أقوى للرعاية الصحية، بما في ذلك المستشفيات المجهزة تجهيزًا جيدًا ومرافق التشخيص. يسمح التشخيص المبكر بالتدخل في الوقت المناسب وبدء العلاج، مما قد يؤدي إلى إبطاء تقدم المرض وتحسين نتائج المرضى. غالبًا ما تحتوي أنظمة الرعاية الصحية المتقدمة على مراكز وعيادات متخصصة مخصصة للأمراض النادرة، مثل دوشين. تقدم هذه المراكز رعاية شاملة، بما في ذلك الوصول إلى متخصصي الرعاية الصحية المتخصصين والعلاج الطبيعي والخدمات الداعمة، والتي يمكن أن تعزز نوعية الحياة لمرضى دوشين.
غالبًا ما توفر أنظمة الرعاية الصحية المتطورة مجموعة واسعة من الخدمات الداعمة، مثل العلاج الطبيعي والمهني ، والأجهزة المساعدة، والاستشارات، والتي يمكن أن تعزز بشكل كبير نوعية الحياة لمرضى دوشين. من المرجح أن تستثمر الحكومات والمستثمرون من القطاع الخاص والمنظمات الخيرية في تطوير الأدوية للأمراض النادرة، مثل دوشين، عندما تكون هناك بنية تحتية راسخة للرعاية الصحية. يمكن أن يدعم هذا الاستثمار الأبحاث والتجارب السريرية وتطوير العلاجات المبتكرة.
ضبط النفس/التحدي
- توافر محدود لعلاجات دوشين بسبب نقص التكنولوجيا المتقدمة
كانت خيارات العلاج المتاحة لمرض دوشين محدودة، مما يترك المرضى وأسرهم مع خيارات قليلة لإبطاء تقدم المرض أو تحسين صحتهم العامة.
غالبًا ما يؤدي التقدم في التكنولوجيا إلى تطوير علاجات متخصصة للغاية ومبتكرة لمرض دوشين، مثل العلاجات الجينية أو أساليب الطب الشخصي. هذه العلاجات المتطورة معقدة في التطوير والتصنيع والإدارة. يتضمن تطوير العلاجات الجينية، على وجه الخصوص، عمليات معقدة وتقنيات تصنيع متخصصة. تتطلب هذه العلاجات تعديل أو استبدال الجينات المعيبة لمعالجة السبب الأساسي لمرض دوشين، مما يؤدي إلى ارتفاع تكاليف العلاج. بالإضافة إلى ذلك، فإن العدد المحدود لمرافق التصنيع القادرة على إنتاج العلاجات الجينية يساهم بشكل أكبر في محدودية توفرها.
إن تعقيد علاجات دوشين المتقدمة قد يجعل تصنيعها وإدارتها أمرًا صعبًا، مما يساهم في محدودية توفرها. وقد تكون هناك حاجة إلى مرافق وخبرات متخصصة للإنتاج، وقد يتطلب إدارة هذه العلاجات متخصصين طبيين، وهو ما قد يكون نادرًا في مناطق معينة.
التطورات الأخيرة
- في مارس 2023، أعلنت شركة Dyne Therapeutics أن DYNE-251، وهو علاج تجريبي لطفرات ضمور العضلات دوشين (DMD) القابلة لتخطي الإكسون 51، حصل على تصنيفات إدارة الغذاء والدواء الأمريكية لدواء يتيم ومرض نادر عند الأطفال. يتم تقييم DYNE-251 في المرحلة 1/2 من التجربة السريرية DELIVER. يساعد هذا المنظمة في زيادة فئة منتجاتها وإجمالي إيراداتها.
- في أغسطس 2022، أعلنت شركة FibroGen, Inc. عن البيانات الأولية من تجربة المرحلة 3 LELANTOS-2 لعقار pamrevlumab لعلاج المرضى المتنقلين المصابين بضمور العضلات الدوشيني الذين يتناولون الكورتيكوستيرويدات الجهازية. وقد ساعد هذا الشركة على تعزيز محفظة خطوط إنتاجها.
نطاق السوق العالمية لعلاج ضمور العضلات دوشين
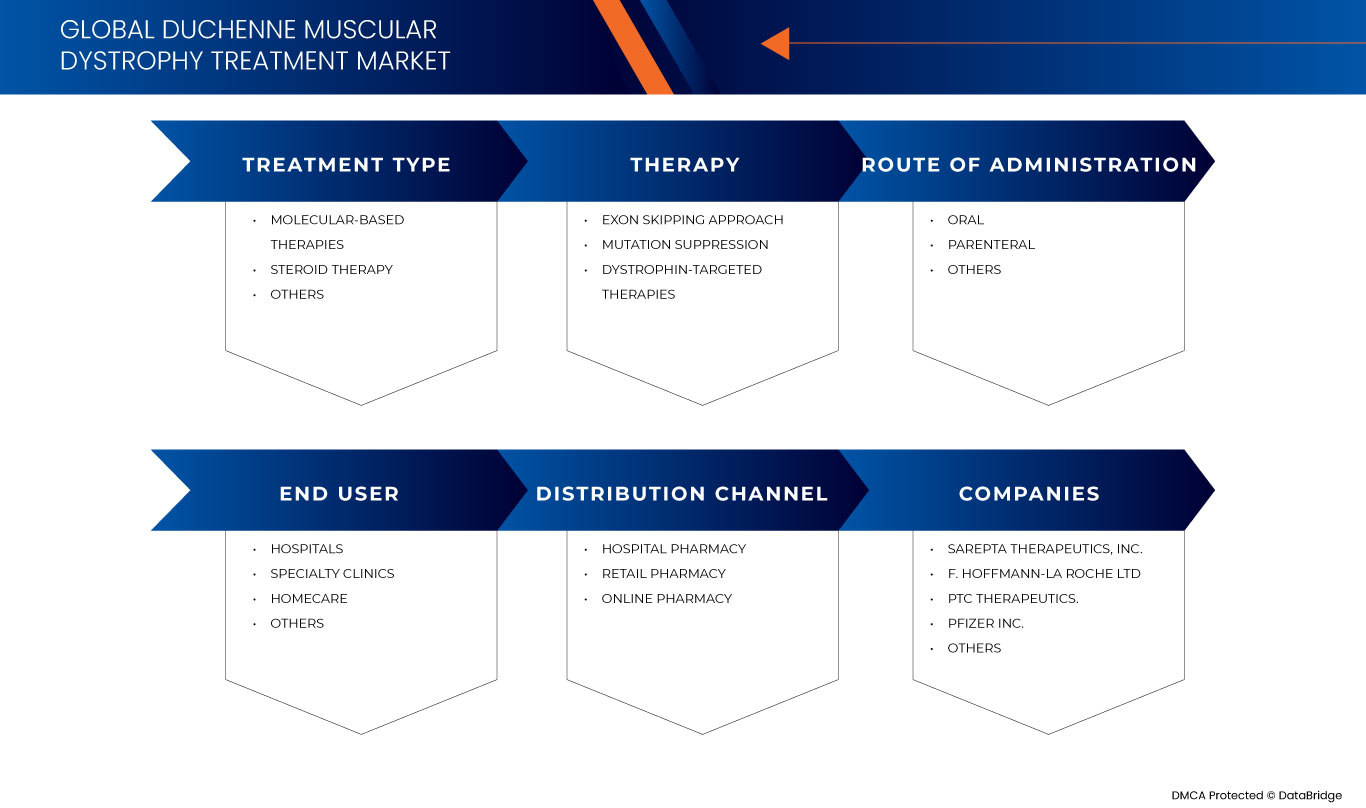
ينقسم سوق علاج ضمور العضلات دوشين العالمي إلى خمسة قطاعات بارزة بناءً على نوع العلاج والعلاج وطريقة الإدارة والمستخدم النهائي وقناة التوزيع. سيساعدك النمو بين هذه القطاعات على تحليل قطاعات النمو الضئيلة في الصناعات وتزويد المستخدمين بنظرة عامة قيمة على السوق ورؤى السوق لمساعدتهم على اتخاذ قرارات استراتيجية لتحديد تطبيقات السوق الأساسية.
نوع العلاج
- العلاجات القائمة على الجزيئات
- العلاج بالستيرويد
- آحرون
على أساس نوع العلاج، يتم تقسيم السوق إلى علاجات تعتمد على الجزيئات، والعلاج بالستيرويد، وغيرها.
مُعَالَجَة
- نهج تخطي الإكسون
- قمع الطفرة
- العلاجات المستهدفة للديستروفين
على أساس العلاج، يتم تقسيم السوق إلى نهج تخطي الإكسون، وقمع الطفرات، والعلاجات المستهدفة للديستروفين.
طريق الإدارة
- شفوي
- الحقن الوريدي
- آحرون
على أساس طريق الإدارة، يتم تقسيم السوق إلى فموي، وحقني، وغيرها.
المستخدم النهائي
- المستشفيات
- الرعاية الصحية المنزلية
- عيادات متخصصة
- آحرون
على أساس المستخدم النهائي، يتم تقسيم السوق إلى المستشفيات والرعاية الصحية المنزلية والعيادات المتخصصة وغيرها.
قناة التوزيع
- صيدلية المستشفى
- صيدلية على الإنترنت
- صيدلية التجزئة
على أساس قناة التوزيع، يتم تقسيم السوق إلى صيدلية المستشفى، والصيدلية عبر الإنترنت، وصيدلية التجزئة .
تحليل إقليمي/رؤى حول سوق علاج ضمور العضلات دوشين العالمي
يتم تقسيم سوق علاج ضمور العضلات دوشين العالمي إلى خمسة قطاعات بارزة بناءً على نوع العلاج والعلاج وطريقة الإدارة والمستخدم النهائي وقناة التوزيع.
الدول التي يغطيها تقرير سوق علاج ضمور العضلات دوشين العالمي هي الولايات المتحدة وكندا والمكسيك وألمانيا وفرنسا والمملكة المتحدة وإيطاليا وإسبانيا وروسيا وتركيا وبلجيكا وهولندا وسويسرا وبقية أوروبا واليابان والصين وكوريا الجنوبية والهند وأستراليا وسنغافورة وتايلاند وماليزيا وإندونيسيا والفلبين وبقية دول آسيا والمحيط الهادئ والبرازيل والأرجنتين وبقية دول أمريكا الجنوبية وجنوب إفريقيا والمملكة العربية السعودية والإمارات العربية المتحدة ومصر وإسرائيل وبقية دول الشرق الأوسط وأفريقيا.
ومن المتوقع أن تهيمن الولايات المتحدة على أمريكا الشمالية بسبب الوعي المتزايد بالمرض وفحوصات الكشف عنه. ومن المتوقع أن تهيمن ألمانيا على أوروبا بسبب انتشار ضمور العضلات المتزايد، واختراق التطورات التكنولوجية الجديدة. ومن المتوقع أن تهيمن الصين على منطقة آسيا والمحيط الهادئ مع توسع المبادرات الاستراتيجية التي تنفذها الجهات الرئيسية في السوق بشكل كبير.
يقدم قسم الدولة في التقرير أيضًا عوامل فردية مؤثرة على السوق والتغيرات في تنظيم السوق التي تؤثر على الاتجاهات الحالية والمستقبلية للسوق. نقاط البيانات مثل تحليل سلسلة القيمة المصب والمصب، والاتجاهات الفنية، وتحليل قوى بورتر الخمس، ودراسات الحالة هي بعض المؤشرات المستخدمة للتنبؤ بسيناريو السوق للدول الفردية. أيضًا، يتم النظر في وجود وتوافر العلامات التجارية العالمية والتحديات التي تواجهها بسبب المنافسة الكبيرة أو النادرة من العلامات التجارية المحلية والمحلية، وتأثير التعريفات الجمركية المحلية، وطرق التجارة أثناء تقديم تحليل تنبؤي لبيانات الدولة.
تحليل المشهد التنافسي وحصة السوق العالمية لعلاج ضمور العضلات دوشين
يوفر المشهد التنافسي العالمي لسوق علاج ضمور العضلات دوشين تفاصيل عن المنافس. تتضمن التفاصيل نظرة عامة على الشركة، والبيانات المالية للشركة، والإيرادات المتولدة، وإمكانات السوق، ومبادرات السوق الجديدة، والحضور العالمي، ومواقع الإنتاج والمرافق، والقدرات الإنتاجية، ونقاط القوة والضعف في الشركة، وإطلاق المنتج، وعرض المنتج ونطاقه، وهيمنة التطبيق. ترتبط نقاط البيانات المذكورة أعلاه فقط بتركيز الشركات فيما يتعلق بالسوق.
بعض اللاعبين الرئيسيين في السوق الذين يعملون في سوق علاج ضمور العضلات دوشين العالمي هم Sarepta Therapeutics، Inc. و GSK plc. و Capricor Therapeutics، Inc. و Dyne Therapeutics، و Solid Biosciences Inc. و BioMarin، و Stealth BioTherapeutics Inc. و Avidity Biosciences، و ReveraGen BioPharma، Inc. و PTC Therapeutics. و NS Pharma، Inc، و ITALFARMACO SpA، و FibroGen، Inc، و SANTHERA PHARMACEUTICALS، و Pfizer Inc. و F. Hoffmann-La Roche Ltd، و Akashi RX، و TAIHO PHARMACEUTICAL CO.، LTD وغيرها.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 TREATMENT TYPE SEGMENT LIFELINE CURVE
2.8 MARKET END USER COVERAGE GRID
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL'S MODEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 PRICING ANALYSIS
5 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: REGULATIONS
5.1 REGULATIONS IN U.S.
5.2 REGULATTIONS IN EUROPE
5.3 REGULATTIONS IN AUSTRALIA
5.4 REGULATIONS IN SOUTH AFRICA
5.5 REGULATIONS IN BRAZIL
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND IMPACT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) DISEASE
6.1.2 INTRODUCTION OF NOVEL THERAPIES FOR DMD DISORDER
6.1.3 RISING AWARENESS FOR DMD TREATMENTS
6.1.4 INCREASE IN THE NUMBER OF CLINICAL TRIALS IS A RECENT TREND
6.2 RESTRAINTS
6.2.1 LIMITED AVAILABILITY OF DMD TREATMENTS DUE TO LACK OF ADVANCE TECHNOLOGY
6.2.2 HIGH TREATMENT COST OF DMD DISORDER
6.3 OPPORTUNITIES
6.3.1 DEVELOPMENT OF HEALTHCARE INFRASTRUCTURE AND INCREASING INVESTMENT
6.3.2 RISING PATIENT INCLINATION TOWARDS PERSONALIZED AND EFFECTIVE THERAPIES
6.3.3 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYERS
6.3.4 SUPPORT FROM PATIENT ADVOCACY GROUPS ON THE REGULATORY APPROVAL PROCESS
6.4 CHALLENGES
6.4.1 LACK OF STANDARDIZATION IN DMD DIAGNOSIS
6.4.2 ETHICAL CONSIDERATIONS RELATED TO PERMANENT ALTERATION OF A PATIENT'S GENETIC CODE
7 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE
7.1 OVERVIEW
7.2 MOLECULAR-BASED THERAPIES
7.2.1 ANTISENSE OLIGONUCLEOTIDE THERAPY
7.2.1.1 EXONDYS 51
7.2.1.2 AMONDYS 45
7.2.1.3 VYONDYS 53
7.3 NONSENSE MUTATION
7.3.1.1 Translarna
7.4 STEROID THERAPY
7.4.1 PREDNISONE
7.4.2 DEFLAZACORT
7.5 OTHERS
8 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY
8.1 OVERVIEW
8.2 EXON SKIPPING APPROACH
8.2.1 MULTI-EXON SKIPPING APPROACH
8.2.2 SINGLE-EXON SKIPPING APPROACH
8.3 MUTATION SUPPRESSION
8.4 DYSTROPHIN-TARGETED THERAPIES
8.4.1 GENE THERAPIES
8.4.2 CELL THERAPIES
8.4.2.1 Gene Editing
8.4.2.2 Gene Addition
9 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
9.1 OVERVIEW
9.2 PARENTERAL
9.3 ORAL
9.4 OTHERS
10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 SPECIALTY CLINICS
10.4 HOMECARE
10.5 OTHERS
11 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 HOSPITAL PHARMACY
11.3 RETAIL PHARMACY
11.4 ONLINE PHARMACY
12 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION
12.1 OVERVIEW
12.2 NORTH AMERICA
12.2.1 U.S.
12.2.2 CANADA
12.2.3 MEXICO
12.3 EUROPE
12.3.1 GERMANY
12.3.2 FRANCE
12.3.3 U.K.
12.3.4 ITALY
12.3.5 SPAIN
12.3.6 RUSSIA
12.3.7 TURKEY
12.3.8 BELGIUM
12.3.9 NETHERLANDS
12.3.10 SWITZERLAND
12.3.11 REST OF EUROPE
12.4 ASIA-PACIFIC
12.4.1 CHINA
12.4.2 JAPAN
12.4.3 INDIA
12.4.4 SOUTH KOREA
12.4.5 AUSTRALIA
12.4.6 SINGAPORE
12.4.7 THAILAND
12.4.8 MALAYSIA
12.4.9 INDONESIA
12.4.10 PHILIPPINES
12.4.11 REST OF ASIA-PACIFIC
12.5 SOUTH AMERICA
12.5.1 BRAZIL
12.5.2 ARGENTINA
12.5.3 REST OF SOUTH AMERICA
12.6 MIDDLE EAST AND AFRICA
12.6.1 SOUTH AFRICA
12.6.2 SAUDI ARABIA
12.6.3 U.A.E
12.6.4 EGYPT
12.6.5 ISRAEL
12.6.6 REST OF MIDDLE EAST AND AFRICA
13 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17 SWOT ANALYSIS
18 COMPANY PROFILES
18.1 SAREPTA THERAPEUTICS, INC.
18.1.1 COMPANY SNAPSHOT
18.1.1 REVENUE ANALYSIS
18.1.2 COMPANY SHARE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 PIPELINE PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 F. HOFFMANN-LA ROCHE LTD
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 PIPELINE PORTFOLIO
18.2.6 RECENT DEVELOPMENTS
18.3 PTC THERAPEUTICS.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 PFIZER INC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PIPELINE PORTFOLIO
18.4.5 PRODUCT PORTFOLIO
18.4.6 RECENT DEVELOPMENT
18.5 AKASHI RX
18.5.1 COMPANY SNAPSHOT
18.5.2 PIPELINE PORTFOLIO
18.5.3 RECENT DEVELOPMENTS
18.6 AVIDITY BIOSCIENCES
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PIPELINE PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 BIOMARIN
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PIPELINE PORTFOLIO
18.7.4 RECENT DEVELOPMENTS
18.8 CAPRICOR THERAPEUTICS, INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PIPELINE PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 DYNE THERAPEUTICS
18.9.1 COMPANY SNAPSHOT
18.9.2 PIPELINE PORTFOLIO
18.9.3 RECENT DEVELOPMENTS
18.1 FIBROGEN, INC.
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PIPELINE PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 ITALFARMACO S.P.A.
18.11.1 COMPANY SNAPSHOT
18.11.2 PIPELINE PORTFOLIO
18.11.3 RECENT DEVELOPMENT
18.12 NS PHARMA, INC.
18.12.1 COMPANY SNAPSHOT
18.12.2 PIPELINE PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 REVERAGEN BIOPHARMA, INC.
18.13.1 COMPANY SNAPSHOT
18.13.2 PIPELINE PORTFOLIO
18.13.3 RECENT DEVELOPMENT
18.14 SANTHERA PHARMACEUTICALS
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PIPELINE PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 TAIHO PHARMACEUTICAL CO., LTD.
18.15.1 COMPANY SNAPSHOT
18.15.2 PIPELINE PORTFOLIO
18.15.3 RECENT DEVELOPMENTS
18.16 SOLID BIOSCIENCES INC.
18.16.1 COMPANY SNAPSHOT
18.16.2 PIPELINE PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 STEALTH BIOTHERAPEUTICS INC
18.17.1 COMPANY SNAPSHOT
18.17.2 PIPELINE PORTFOLIO
18.17.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
List of Table
TABLE 1 LIST OF PRICES FOR APPROVED DRUGS OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
TABLE 2 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE , 2021-2030 (USD THOUSAND)
TABLE 3 GLOBAL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 4 GLOBAL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 5 GLOBAL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 GLOBAL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 7 GLOBAL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 8 GLOBAL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 9 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 11 GLOBAL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 12 GLOBAL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 13 GLOBAL MUTATION SUPPRESSION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 14 GLOBAL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 15 GLOBAL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 16 GLOBAL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 17 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION , 2021-2030 (USD THOUSAND)
TABLE 18 GLOBAL PARENTERAL IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 19 GLOBAL ORAL IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 20 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 21 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USERS, 2021-2030 (USD THOUSAND)
TABLE 22 GLOBAL HOSPITALS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 23 GLOBAL SPECIALITY CLINICS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 24 GLOBAL HOMECARE IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 25 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 26 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 27 GLOBAL HOSPITAL PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 28 GLOBAL RETAIL PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 29 GLOBAL ONLINE PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 30 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 31 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND) COUNTRY
TABLE 32 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 33 NORTH AMERICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 34 NORTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 35 NORTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 36 NORTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 37 NORTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 38 NORTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 39 NORTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 40 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 41 NORTH AMERICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 42 NORTH AMERICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 43 NORTH AMERICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 44 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 45 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 46 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 47 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 48 U.S. MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 49 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 50 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 51 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 52 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 53 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 54 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 55 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 56 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 57 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 58 U.S.DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 59 U.S. EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 60 U.S. DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 61 U.S. CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 62 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 63 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 64 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 65 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 66 CANADA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 67 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 68 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 69 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 70 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 71 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 72 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 73 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 74 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 75 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 76 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 77 CANADA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 78 CANADA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 79 CANADA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 80 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 81 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 82 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 83 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 84 MEXICO MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 85 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 86 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 87 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 88 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 89 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 90 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 91 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 92 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 93 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 94 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 95 MEXICO EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 96 MEXICO DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 97 MEXICO CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 98 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 99 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 100 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 101 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 102 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 103 EUROPE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 104 EUROPE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 105 EUROPE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 106 EUROPE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 107 EUROPE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 108 EUROPE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 109 EUROPE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 110 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 111 EUROPE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 112 EUROPE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 113 EUROPE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 114 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 115 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 116 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 117 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 118 GERMANY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 119 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 120 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 121 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 122 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 123 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 124 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 125 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 126 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 127 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 128 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 129 GERMANY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 130 GERMANY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 131 GERMANY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 132 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 133 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 134 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 135 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 136 FRANCE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 137 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 138 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 139 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 140 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 141 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 142 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 143 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 144 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 145 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 146 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 147 FRANCE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 148 FRANCE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 149 FRANCE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 150 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 151 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 152 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 153 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 154 U.K. MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 155 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 156 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 157 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 158 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 159 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 160 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 161 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 162 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 163 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 164 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 165 U.K. EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 166 U.K. DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 167 U.K. CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 168 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 169 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 170 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 171 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 172 ITALY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 173 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 174 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 175 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 176 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 177 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 178 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 179 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 180 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 181 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 182 ITALYDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 183 ITALY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 184 ITALY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 185 ITALY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 186 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 187 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 188 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 189 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 190 SPAIN MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 191 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 192 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 193 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 194 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 195 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 196 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 197 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 198 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 199 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 200 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 201 SPAIN EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 202 SPAIN DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 203 SPAIN CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 204 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 205 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 206 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 207 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 208 RUSSIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 209 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 210 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 211 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 212 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 213 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 214 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 215 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 216 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 217 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 218 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 219 RUSSIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 220 RUSSIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 221 RUSSIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 222 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 223 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 224 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 225 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 226 TURKEY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 227 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 228 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 229 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 230 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 231 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 232 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 233 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 234 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 235 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 236 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 237 TURKEY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 238 TURKEY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 239 TURKEY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 240 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 241 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 242 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 243 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 244 BELGIUM MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 245 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 246 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 247 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 248 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 249 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 250 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 251 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 252 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 253 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 254 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 255 BELGIUM EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 256 BELGIUM DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 257 BELGIUM CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 258 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 259 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 260 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 261 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 262 NETHERLANDS MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 263 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 264 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 265 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 266 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 267 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 268 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 269 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 270 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 271 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 272 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 273 NETHERLANDS EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 274 NETHERLANDS DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 275 NETHERLANDS CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 276 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 277 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 278 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 279 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 280 SWITZERLAND MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 281 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 282 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 283 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 284 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 285 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 286 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 287 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 288 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 289 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 290 SWITZERLANDDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 291 SWITZERLAND EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 292 SWITZERLAND DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 293 SWITZERLAND CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 294 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 295 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 296 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 297 REST OF EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 298 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 299 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 300 ASIA-PACIFIC MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 301 ASIA-PACIFIC ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 302 ASIA-PACIFIC ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 303 ASIA-PACIFIC NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 304 ASIA-PACIFIC NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 305 ASIA-PACIFIC STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 306 ASIA-PACIFIC STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 307 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 308 ASIA-PACIFIC EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 309 ASIA-PACIFIC DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 310 ASIA-PACIFIC CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 311 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 312 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 313 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 314 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 315 CHINA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 316 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 317 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 318 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 319 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 320 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 321 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 322 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 323 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 324 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 325 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 326 CHINA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 327 CHINA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 328 CHINA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 329 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 330 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 331 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 332 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 333 JAPAN MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 334 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 335 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 336 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 337 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 338 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 339 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 340 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 341 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 342 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 343 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 344 JAPAN EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 345 JAPAN DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 346 JAPAN CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 347 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 348 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 349 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 350 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 351 INDIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 352 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 353 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 354 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 355 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 356 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 357 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 358 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 359 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 360 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 361 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 362 INDIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 363 INDIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 364 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 365 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 366 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 367 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 368 SOUTH KOREA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 369 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 370 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 371 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 372 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 373 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 374 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 375 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 376 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 377 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 378 SOUTH KOREADUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 379 SOUTH KOREA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 380 SOUTH KOREA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 381 SOUTH KOREA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 382 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 383 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 384 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 385 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 386 AUSTRALIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 387 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 388 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 389 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 390 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 391 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 392 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 393 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 394 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 395 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 396 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 397 AUSTRALIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 398 AUSTRALIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 399 AUSTRALIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 400 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 401 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 402 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 403 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 404 SINGAPORE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 405 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 406 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 407 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 408 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 409 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 410 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 411 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 412 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 413 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 414 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 415 SINGAPORE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 416 SINGAPORE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 417 SINGAPORE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 418 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 419 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 420 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 421 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 422 THAILAND MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 423 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 424 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 425 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 426 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 427 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 428 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 429 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 430 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 431 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 432 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 433 THAILAND EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 434 THAILAND DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 435 THAILAND CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 436 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 437 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 438 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 439 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 440 MALAYSIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 441 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 442 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 443 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 444 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 445 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 446 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 447 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 448 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 449 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 450 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 451 MALAYSIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 452 MALAYSIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 453 MALAYSIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 454 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 455 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 456 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 457 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 458 INDONESIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 459 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 460 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 461 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 462 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 463 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 464 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 465 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 466 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 467 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 468 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 469 INDONESIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 470 INDONESIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 471 INDONESIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 472 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 473 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 474 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 475 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 476 PHILIPPINES MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 477 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 478 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 479 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 480 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 481 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 482 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 483 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 484 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 485 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 486 PHILIPPINESDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 487 PHILIPPINES EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 488 PHILIPPINES DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 489 PHILIPPINES CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 490 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 491 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 492 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 493 REST OF ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 494 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 495 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 496 SOUTH AMERICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 497 SOUTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 498 SOUTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 499 SOUTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 500 SOUTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 501 SOUTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 502 SOUTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 503 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 504 SOUTH AMERICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 505 SOUTH AMERICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 506 SOUTH AMERICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 507 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 508 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 509 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 510 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 511 BRAZIL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 512 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 513 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 514 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 515 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 516 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 517 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 518 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 519 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 520 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 521 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 522 BRAZIL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 523 BRAZIL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 524 BRAZIL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 525 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 526 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 527 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 528 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 529 ARGENTINA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 530 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 531 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 532 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 533 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 534 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 535 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 536 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 537 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 538 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 539 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 540 ARGENTINA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 541 ARGENTINA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 542 ARGENTINA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 543 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 544 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 545 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 546 REST OF SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 547 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 548 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 549 MIDDLE EAST AND AFRICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 550 MIDDLE EAST AND AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 551 MIDDLE EAST AND AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 552 MIDDLE EAST AND AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 553 MIDDLE EAST AND AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 554 MIDDLE EAST AND AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 555 MIDDLE EAST AND AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 556 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 557 MIDDLE EAST AND AFRICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 558 MIDDLE EAST AND AFRICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 559 MIDDLE EAST AND AFRICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 560 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 561 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 562 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 563 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 564 SOUTH AFRICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 565 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 566 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 567 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 568 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 569 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 570 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 571 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 572 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 573 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 574 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 575 SOUTH AFRICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 576 SOUTH AFRICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 577 SOUTH AFRICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 578 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 579 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 580 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 581 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 582 SAUDI ARABIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 583 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 584 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 585 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 586 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 587 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 588 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 589 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 590 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 591 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 592 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 593 SAUDI ARABIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 594 SAUDI ARABIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 595 SAUDI ARABIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 596 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 597 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 598 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 599 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 600 U.A.E MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 601 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 602 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 603 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 604 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 605 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 606 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 607 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 608 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 609 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 610 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 611 U.A.E EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 612 U.A.E DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 613 U.A.E CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 614 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 615 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 616 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 617 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 618 EGYPT MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 619 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 620 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 621 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 622 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 623 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 624 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 625 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 626 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 627 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 628 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 629 EGYPT EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 630 EGYPT DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 631 EGYPT CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 632 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 633 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 634 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 635 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 636 ISRAEL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 637 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 638 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 639 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 640 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 641 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 642 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 643 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 644 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 645 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 646 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 647 ISRAEL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 648 ISRAEL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 649 ISRAEL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 650 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 651 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 652 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 653 REST OF MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
List of Figure
FIGURE 1 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: SEGMENTATION
FIGURE 2 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE AND IMPACT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) AND INTRODUCTION OF NOVEL THERAPIES FOR DMD DISORDER ARE EXPECTED TO DRIVE THE GROWTH OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET FROM 2023 TO 2030
FIGURE 12 MOLECULAR-BASED THERAPIES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
FIGURE 14 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE , 2022
FIGURE 15 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE, 2023-2030 (USD THOUSAND)
FIGURE 16 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE , CAGR (2023-2030)
FIGURE 17 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE, LIFELINE CURVE
FIGURE 18 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, 2022
FIGURE 19 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, 2023-2030 (USD THOUSAND)
FIGURE 20 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, CAGR (2023-2030)
FIGURE 21 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 22 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 23 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD THOUSAND)
FIGURE 24 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 25 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 26 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, 2022
FIGURE 27 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, 2023-2030 (USD THOUSAND)
FIGURE 28 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD THOUSAND)
FIGURE 32 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET SNAPSHOT
FIGURE 35 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 36 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 37 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 38 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.