سوق الخلايا الجذعية المحفزة متعددة القدرات في أوروبا (iPSCs)، حسب مصدر الخلية (خلايا الجلد وخلايا الدم)، النوع (IPSCs البشرية وIPSCs الفأرية)، المنتج (الأدوات والمواد الاستهلاكية والمجموعات والخدمات)، التطبيقات (البحث الأكاديمي، الطب التجديدي، العلاج الخلوي، فحص السموم، اكتشاف وتطوير الأدوية، نمذجة الأمراض، بنوك الخلايا الجذعية، الطباعة الحيوية ثلاثية الأبعاد وغيرها)، المستخدم النهائي (شركات التكنولوجيا الحيوية والأدوية، مختبرات الأبحاث، مختبرات التشخيص وغيرها)، قناة التوزيع (العطاءات المباشرة ومبيعات التجزئة)، الدولة (ألمانيا والمملكة المتحدة وإيطاليا وفرنسا وإسبانيا وسويسرا وروسيا وتركيا وبلجيكا وهولندا وبقية أوروبا) - اتجاهات الصناعة والتوقعات حتى عام 2029.
تحليل السوق والرؤى: سوق الخلايا الجذعية المحفزة متعددة القدرات في أوروبا (iPSCs)
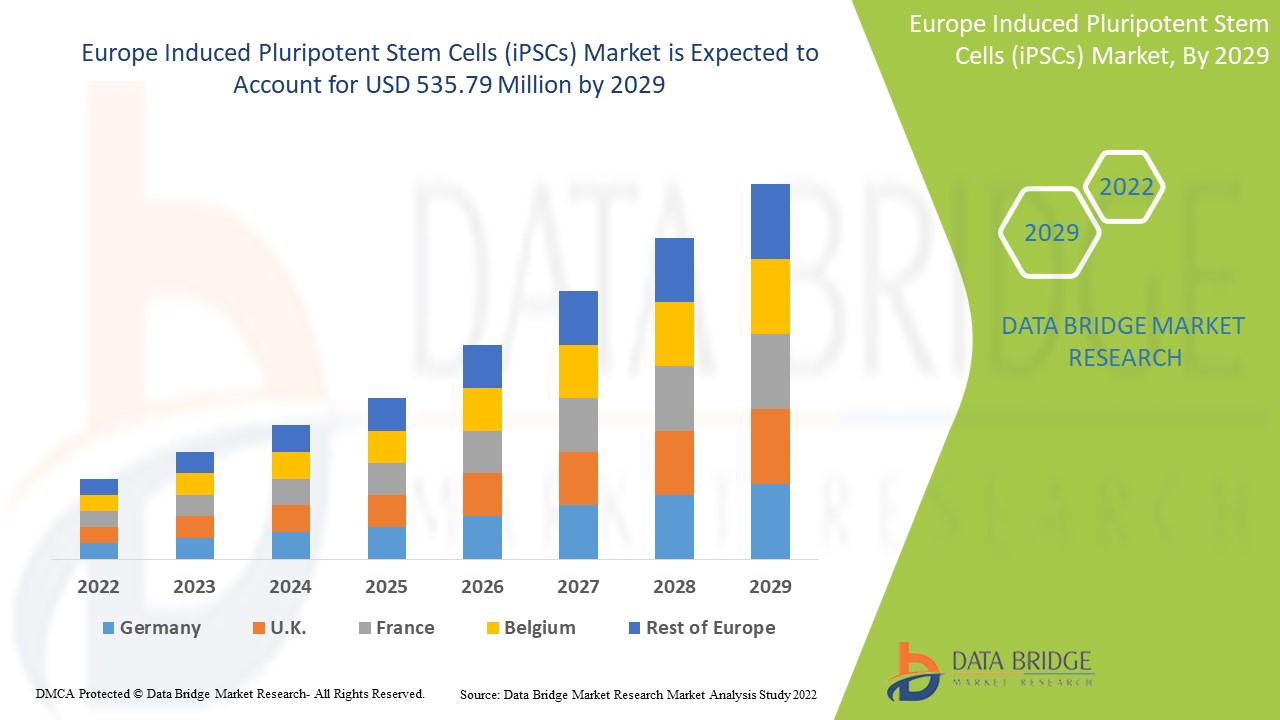
من المتوقع أن يكتسب سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) نموًا في السوق في الفترة المتوقعة من 2022 إلى 2029. تحلل Data Bridge Market Research أن السوق ينمو بمعدل نمو سنوي مركب بنسبة 8.9٪ في الفترة المتوقعة من 2022 إلى 2029 ومن المتوقع أن يصل إلى 535.79 مليون دولار أمريكي بحلول عام 2029. تعمل أنشطة البحث المتزايدة حول علاجات الخلايا الجذعية كمحرك لنمو سوق الخلايا الجذعية متعددة القدرات المستحثة (iPSCs).
الخلايا الجذعية متعددة القدرات المستحثة هي نوع من الخلايا المشتقة من الأنسجة الجسدية البالغة والتي أعيد برمجتها بمجموعة من الجينات والعوامل لاكتساب طبيعة متعددة القدرات. تتم إضافة جينات وعوامل معينة للحصول على خصائص محددة للخلايا الجذعية الجنينية. الخلايا متعددة القدرات المستحثة متطابقة تقريبًا مع الخلايا المانحة، فهي تساعد في نمذجة المرض. تُستخدم الفيروسات الرجعية عادةً كناقلات لإعادة برمجة الخلايا الجذعية متعددة القدرات المستحثة. التطبيقات الرئيسية للخلايا الجذعية متعددة القدرات المستحثة هي في نمذجة المرض واكتشاف الأدوية وتطويرها ودراسات السمية والعلاجات الجينية. تُستخدم على نطاق واسع في علاج أمراض القلب والأوعية الدموية ومرض السكري وأنواع مختلفة من السرطان. تُظهر الخلايا الجذعية متعددة القدرات المستحثة لدى البشر الخصائص ذات الصلة بالمرض لأنها تحمل النمط الجيني المحدد للمرض، وبالتالي تمكن من خيارات علاجية جديدة بطريقة خاصة بالمريض.
إن زيادة استخدام العلاج بالخلايا الجذعية، ونمو قطاع التكنولوجيا الحيوية مع تحسن الاستثمار وارتفاع معدل انتشار الأمراض المزمنة، كلها عوامل تعمل كمحرك لسوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs). ومن بين العوامل الأخرى التي من المتوقع أن تدفع نمو سوق الخلايا الجذعية المحفزة متعددة القدرات في أوروبا، النطاق الواسع للتطبيقات السريرية للخلايا الجذعية المحفزة متعددة القدرات والتقدم التكنولوجي الناشئ للخلايا الجذعية المحفزة متعددة القدرات.
ومع ذلك، فإن العوامل مثل التكلفة العالية المرتبطة بعلاجات الخلايا الجذعية، وتوافر البدائل لعلاج الأورام تعيق نمو سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs). من ناحية أخرى، فإن العدد المتزايد من المنتجات المتاحة، والاهتمام المتزايد بالطب الشخصي والارتفاع في الإنفاق على الرعاية الصحية بمثابة فرصة لنمو سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs). إن القواعد واللوائح الصارمة وعدم الاستقرار الجيني للخلايا الجذعية متعددة القدرات المستحثة في أوروبا هي التحدي الرئيسي الذي يواجه سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs).
يقدم تقرير سوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs) تفاصيل عن حصة السوق والتطورات الجديدة وتحليل خط أنابيب المنتجات وتأثير اللاعبين المحليين والمحليين في السوق وتحليل الفرص من حيث جيوب الإيرادات الناشئة والتغييرات في لوائح السوق وموافقات المنتجات والقرارات الاستراتيجية وإطلاق المنتجات والتوسعات الجغرافية والابتكارات التكنولوجية في السوق. لفهم تحليل وسيناريو سوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs)، اتصل بـ Data Bridge Market Research للحصول على موجز محلل، وسيساعدك فريقنا في إنشاء حل تأثير الإيرادات لتحقيق هدفك المنشود.
نطاق سوق الخلايا الجذعية متعددة القدرات المستحثة (iPSCs) وحجم السوق
يتم تقسيم سوق الخلايا الجذعية متعددة القدرات المستحثة (iPSCs) على أساس مصدر الخلية والنوع والمنتج والتطبيقات والمستخدمين النهائيين وقناة التوزيع. يساعدك النمو بين القطاعات على تحليل جيوب النمو والاستراتيجيات المتخصصة للتعامل مع السوق وتحديد مجالات التطبيق الأساسية لديك والفرق في أسواقك المستهدفة.
يتم تصنيف سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) إلى ستة قطاعات بارزة بناءً على مصدر الخلية والنوع والمنتج والتطبيقات والمستخدمين النهائيين وقناة التوزيع.
- على أساس مصدر الخلايا، يتم تقسيم سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) إلى خلايا الجلد وخلايا الدم. في عام 2022، من المتوقع أن تهيمن شريحة خلايا الجلد على السوق بسبب التوافر الواسع لمصادر خلايا الجلد والوعي العالي بها.
- على أساس النوع، يتم تقسيم سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) إلى IPSC البشرية و IPSCs الفأرية. في عام 2022، من المتوقع أن تهيمن شريحة IPSC البشرية على السوق بسبب توسع عدد كبار السن وزيادة عدد التجارب السريرية في فرنسا.
- على أساس المنتج، يتم تقسيم سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) إلى أدوات ومواد استهلاكية ومجموعات وخدمات. في عام 2022، من المتوقع أن تهيمن شريحة المواد الاستهلاكية والمجموعات على السوق بسبب زيادة البحث والتطوير في المملكة المتحدة بسبب وجود خبراء في علم الأحياء التنموي والتكاثري.
- على أساس التطبيق، يتم تقسيم سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) إلى البحث الأكاديمي والطب التجديدي والعلاج الخلوي وفحص السموم واكتشاف الأدوية وتطويرها ونمذجة الأمراض وبنوك الخلايا الجذعية والطباعة الحيوية ثلاثية الأبعاد وغيرها. في عام 2022، من المتوقع أن يهيمن قطاع اكتشاف الأدوية وتطويرها على السوق حيث عزز الاستعانة بمصادر خارجية لتجارب iPSCs في النهاية عملية الموافقة على الأدوية.
- على أساس المستخدمين النهائيين، يتم تقسيم سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) إلى شركات التكنولوجيا الحيوية والأدوية ومختبرات الأبحاث ومختبرات التشخيص وغيرها. في عام 2022، من المتوقع أن تهيمن شريحة شركات التكنولوجيا الحيوية والأدوية على السوق حيث أصبحت المنطقة مؤخرًا مركزًا لشركات الأدوية الحيوية الصغيرة والكبيرة وأصبحت تعتمد على قطاع منظمة أبحاث العقود والخدمات السريرية الأخرى لعمليات البحث والتطوير.
- على أساس قناة التوزيع، يتم تقسيم سوق الخلايا الجذعية متعددة القدرات المستحثة في أوروبا (iPSCs) إلى العطاء المباشر ومبيعات التجزئة. في عام 2022، من المتوقع أن تهيمن شريحة العطاء المباشر على السوق بسبب العدد الكبير من مصادر التسليم.
تحليل على مستوى الدولة لسوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs)
يتم تحليل سوق الخلايا الجذعية متعددة القدرات المستحثة (iPSCs) ويتم توفير معلومات حجم السوق حسب البلد ومصدر الخلية والنوع والمنتج والتطبيقات والمستخدمين النهائيين وقناة التوزيع كما هو مذكور أعلاه.
الدول التي يغطيها تقرير سوق الخلايا الجذعية متعددة القدرات المستحثة (iPSCs) هي المملكة المتحدة وألمانيا وفرنسا وإسبانيا وإيطاليا وهولندا وسويسرا وروسيا وتركيا والنمسا وأيرلندا وبقية أوروبا.
من المتوقع أن ينمو قطاع الخلايا الجذعية المحفزة متعددة القدرات البشرية في ألمانيا بأعلى معدل نمو في الفترة المتوقعة من 2022 إلى 2029 بسبب الاستخدام المتزايد لتكنولوجيا الخلايا الجذعية. يهيمن قطاع الخلايا الجذعية المحفزة متعددة القدرات البشرية في فرنسا على السوق الأوروبية بسبب زيادة حالات الأمراض المزمنة والتبني العالي لمصادر الخلايا الجذعية للحصول على علاجات أفضل. تقود المملكة المتحدة نمو السوق ويهيمن قطاع الخلايا الجذعية المحفزة متعددة القدرات البشرية في هذا البلد بسبب العدد المتزايد من مراكز التكنولوجيا الحيوية وأنشطة البحث.
كما يوفر قسم الدولة في التقرير عوامل التأثير الفردية على السوق والتغييرات في التنظيم في السوق محليًا والتي تؤثر على الاتجاهات الحالية والمستقبلية للسوق. تعد نقاط البيانات مثل المبيعات الجديدة ومبيعات الاستبدال والتركيبة السكانية للدولة والقوانين التنظيمية ورسوم الاستيراد والتصدير من بين المؤشرات الرئيسية المستخدمة للتنبؤ بسيناريو السوق للدول الفردية. كما يتم النظر في وجود وتوافر العلامات التجارية الأوروبية والتحديات التي تواجهها بسبب المنافسة الكبيرة أو النادرة من العلامات التجارية المحلية والمحلية وتأثير قنوات المبيعات أثناء تقديم تحليل توقعات لبيانات الدولة.
إن الأنشطة الاستراتيجية المتنامية التي يقوم بها كبار اللاعبين في السوق لتعزيز الوعي بعلاج الخلايا الجذعية المحفزة متعددة القدرات (iPSCs) تعمل على تعزيز نمو سوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs).
كما يوفر لك سوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs) تحليلاً تفصيلياً للسوق لكل نمو في سوق معين. بالإضافة إلى ذلك، يوفر معلومات تفصيلية حول استراتيجية اللاعبين في السوق ووجودهم الجغرافي. البيانات متاحة للفترة التاريخية من 2011 إلى 2020.
تحليل المنافسة وحصة سوق الخلايا الجذعية متعددة القدرات المستحثة (iPSCs)
يوفر المشهد التنافسي لسوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs) تفاصيل حسب المنافس. تتضمن التفاصيل نظرة عامة على الشركة، والمالية، والإيرادات المتولدة، وإمكانات السوق، والاستثمار في البحث والتطوير، ومبادرات السوق الجديدة، ومواقع الإنتاج والمرافق، ونقاط القوة والضعف في الشركة، وإطلاق المنتج، وخطوط أنابيب تجارب المنتجات، وموافقات المنتج، وبراءات الاختراع، وعرض المنتج ونطاقه، وهيمنة التطبيق، ومنحنى شريان الحياة التكنولوجي. ترتبط نقاط البيانات المذكورة أعلاه فقط بتركيز الشركة فيما يتعلق بسوق الخلايا الجذعية المحفزة متعددة القدرات (iPSCs).
الشركات الكبرى التي تتعامل في مجال الخلايا الجذعية متعددة القدرات المستحثة (iPSCs) هي Thermo Fisher Scientific Inc. وFUJIFILM Corporation وLumaCyte وHorizon Discovery Ltd. وTakara Bio Inc. وLonza. وEvotec SE. وAxol Bioscience Ltd. وR & D Systems, Inc. وCharles River Laboratories International, Inc. وCorning Incorporated وREPROCELL Inc. وMerck KGaA وغيرها من الشركات المحلية. يفهم محللو DBMR نقاط القوة التنافسية ويقدمون تحليلاً تنافسيًا لكل منافس على حدة.
كما يتم أيضًا إبرام العديد من العقود والاتفاقيات من قبل الشركات في جميع أنحاء العالم والتي تعمل أيضًا على تسريع سوق الخلايا الجذعية متعددة القدرات (iPSCs).
على سبيل المثال،
- في فبراير 2021، أعلنت شركة Thermo Fisher Scientific Inc. عن فوزها بست جوائز في حفل توزيع جوائز CMO Leadership السنوي. تُقدَّم الجوائز من Life Science Leader وOutsourced Pharma، تكريمًا لأفضل شركاء التصنيع التعاقدي وفقًا لتقييم شركات الأدوية الحيوية والتكنولوجيا الحيوية. ومن المتوقع أن يعزز هذا التقدير من بصمات الشركة في السوق الأوروبية ويؤدي إلى زيادة نمو الشركة في السنوات القادمة.
- في يونيو 2020، تعاونت شركة LumaCyte مع شركة Catalent، وهي الشركة الأوروبية التي تقدم تقنيات التوصيل المتقدمة وحلول التطوير والتصنيع للأدوية والمستحضرات البيولوجية وعلاجات الخلايا والجينات ومنتجات صحة المستهلك. ساعد هذا التعاون في توسيع نطاق منتج تقنية الخلايا الجذعية Radiance الخاص بالشركة وتطبيقاته.
إن التعاون وإطلاق المنتج وتوسيع الأعمال والجوائز والتقدير والمشاريع المشتركة والاستراتيجيات الأخرى التي يتبعها اللاعب في السوق تعمل على تعزيز بصمة الشركة في سوق مضخات التسريب البيطرية مما يوفر أيضًا فائدة لنمو أرباح المنظمة.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CELL SOURCE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
5 EUROPE MEDICAL CARTS MARKET: REGULATIONS
5.1 REGULATION IN U.S.
5.2 REGULATION IN CANADA
5.3 REGULATION IN EUROPE
5.4 REGULATION IN INDIA
5.5 REGUALTION IN JAPAN
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 WIDE RANGE OF CLINICAL APPLICATION OF INDUCED PLURIPOTENT STEM CELLS
6.1.2 EMERGING TECHNOLOGICAL ADVANTAGES OF IPSCS
6.1.3 RISING PREVALENCE OF SEVERAL CHRONIC DISEASES
6.1.4 INCREASING ADOPTION OF STEM CELL THERAPY
6.1.5 GROWING BIOTECHNOLOGY SECTOR WITH BETTER INVESTMENT
6.2 RESTRAINT
6.2.1 HIGH COST ASSOCIATED WITH STEM CELL THERAPIES AND LARGE-SCALE APPLICATIONS OF IPSCS
6.2.2 AVAILABILITY OF ALTERNATIVES FOR TUMOR TREATMENT
6.2.3 ADVERSE EFFECTS OF STEM CELL TRANSPLANTS
6.3 OPPORTUNITIES
6.3.1 INCREASING NUMBER OF PIPELINE PRODUCTS
6.3.2 INCREASING INTEREST OF PERSONALIZED MEDICINE
6.3.3 SURGE IN HEALTHCARE EXPENDITURE
6.3.4 STRATEGIC INITIATIVES BY KEY MARKET PLAYERS
6.4 CHALLENGES
6.4.1 GENOMIC INSTABILITY OF IPSCS IS THE KEY MARKET CHALLENGE
6.4.2 LACK OF SKILLED PROFESSIONALS
6.4.3 STRINGENT REGULATORY FRAMEWORK
7 IMPACT OF COVID-19 ON THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
7.1 IMPACT ON PRICE
7.2 IMPACT ON DEMAND
7.3 IMPACT ON SUPPLY CHAIN
7.4 STRATEGIC DECISIONS BY MANUFACTURERS
7.5 CONCLUSION
8 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE
8.1 OVERVIEW
8.2 SKIN CELLS
8.2.1 FIBROBLAST
8.2.2 KERATINOCYTES
8.2.3 ADIPOSE DERIVED STEM CELLS
8.2.4 HEPATOCYTES
8.2.5 MELANOCYTES
8.2.6 NEURAL STEM CELLS
8.2.7 OTHERS
8.3 BLOOD CELLS
8.3.1 PERIPHERAL BLOOD
8.3.2 CORD BLOOD ENDOTHELIAL CELLS
8.3.3 CORD BLOOD STEM CELLS
8.3.4 OTHERS
9 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE
9.1 OVERVIEW
9.2 HUMAN IPSCS
9.3 MOUSE IPSCS
10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT
10.1 OVERVIEW
10.2 CONSUMABLES & KITS
10.2.1 REPROGRAMMING KITS
10.2.2 MEDIA
10.2.3 TRANSFECTION KITS
10.2.4 CELL IDENTIFICATION KITS
10.2.5 ACCESSORIES
10.2.6 OTHERS
10.3 SERVICES
10.4 INSTRUMENTS
10.4.1 IMAGING SYSTEMS
10.4.2 ELECTROPORATION DEVICE
10.4.3 INCUBATORS
10.4.4 OTHERS
11 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 DRUG DISCOVERY AND DEVELOPMENT
11.3 ACADEMIC RESEARCH
11.4 DISEASE MODELLING
11.5 CELLULAR THERAPY
11.6 REGENERATIVE MEDICINE
11.7 TOXICOLOGY SCREENING
11.8 STEM CELL BANKING
11.9 3D BIOPRINTING
11.1 OTHERS
12 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER
12.1 OVERVIEW
12.2 BIOTECHNOLOGY & PHARMACEUTICAL COMPANIES
12.3 RESEARCH LABORATORIES
12.4 DIAGNOSTIC LABORATORIES
12.5 OTHERS
13 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
14 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 FRANCE
14.1.3 U.K.
14.1.4 ITALY
14.1.5 RUSSIA
14.1.6 SPAIN
14.1.7 TURKEY
14.1.8 NETHERLANDS
14.1.9 SWITZERLAND
14.1.10 BELGIUM
14.1.11 REST OF EUROPE
15 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 FUJIFILM CORPORATION
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.1.5.1 ACQUISITION
17.2 THERMO FISHER SCIENTIFIC INC.
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.2.5.1 EVENT
17.2.5.2 ACQUISITION
17.3 LONZA.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.3.5.1 EXPANSION
17.4 MERCK KGAA
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.4.5.1 AGREEMENT
17.5 EVOTEC SE.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENTS
17.5.5.1 AGREEMENT
17.5.5.2 COLLABORATION
17.6 APPLIED STEMCELL.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENTS
17.6.3.1 PRODUCT LAUNCH
17.7 AXOL BIOSCIENCE LTD.
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.7.3.1 MERGER
17.7.3.2 PRODUCT LAUNCH
17.8 CELL APPLICATIONS, INC.
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.8.3.1 PARTNERSHIP
17.9 CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENT
17.9.4.1 ACQUISITION
17.1 CITIUS PHARMACEUTICALS, INC.
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.10.3.1 AGREEMENT
17.11 CORNING INCORPORATED
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.11.4.1 AGREEMENT
17.12 FATE THERAPEUTICS
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENT
17.12.3.1 CLINICAL TRIAL
17.13 GENECOPOEIA, INC.
17.13.1 COMPANY SNAPSHOT
17.13.2 PRODUCT PORTFOLIO
17.13.3 RECENT DEVELOPMENT
17.14 HOPSTEM BIOTECHNOLOGY LLC.
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.14.3.1 PARTNERSHIP
17.15 HORIZON DISCOVERY LTD.
17.15.1 COMPANY SNAPSHOT
17.15.2 PRODUCT PORTFOLIO
17.15.3 RECENT DEVELOPMENT
17.16 LUMACYTE
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENT
17.16.3.1 COLLABORATION
17.17 R & D SYSTEMS, INC.
17.17.1 COMPANY SNAPSHOT
17.17.2 REVENUE ANALYSIS
17.17.3 PRODUCT PORTFOLIO
17.17.4 RECENT DEVELOPMENT
17.18 REPROCELL INC.
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENTS
17.18.3.1 COLLABORATION
17.18.3.2 FACILITY EXPANSION
17.18.3.3 SERVICE LAUNCH
17.19 TAKARA BIO INC.
17.19.1 COMPANY SNAPSHOT
17.19.2 REVENUE ANALYSIS
17.19.3 PRODUCT PORTFOLIO
17.19.4 RECENT DEVELOPMENTS
17.19.4.1 NEW FACILITY LAUNCH
17.2 UNIVERSAL CELLS INC. (AN ASTELLAS COMPANY)
17.20.1 COMPANY SNAPSHOT
17.20.2 REVENUE ANALYSIS
17.20.3 PRODUCT PORTFOLIO
17.20.4 RECENT DEVELOPMENT
17.20.4.1 ACQUISITION
18 QUESTIONNAIRE
19 RELATED REPORTS
List of Table
TABLE 1 NEW CANCER CASES, AGES 85+, IN THE U.S.
TABLE 2 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 3 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 7 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 8 EUROPE HUMAN IPSCS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE MOUSE IPSCS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 11 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 13 EUROPE SERVICES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 16 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE DRUG DISCOVERY AND DEVELOPMENT IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ACADEMIC RESEARCH IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE DISEASE MODELLING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE CELLULAR THERAPY IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE REGENERATIVE MEDICINE IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE TOXICOLOGY SCREENING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE STEM CELL BANKING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE 3D BIOPRINTING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE OTHERS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 27 EUROPE BIOTECHNOLOGY & PHARMACEUTICAL COMPANIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE RESEARCH LABORATORIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE DIAGNOSTIC LABORATORIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE OTHERS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 32 EUROPE DIRECT TENDER IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE RETAIL SALES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 35 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 36 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 37 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 38 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 39 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 40 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 41 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 42 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 43 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 46 GERMANY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 47 GERMANY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 48 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 50 GERMANY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 51 GERMANY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 52 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 53 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 54 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 55 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 56 FRANCE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 57 FRANCE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 58 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 59 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 60 FRANCE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 61 FRANCE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 62 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 63 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 64 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 65 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 66 U.K. SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 67 U.K. BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 68 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 69 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 70 U.K. INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 71 U.K. CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 72 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 73 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 76 ITALY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 77 ITALY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 78 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 80 ITALY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 81 ITALY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 82 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 83 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 84 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 85 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 86 RUSSIA SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 87 RUSSIA BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 88 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 89 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 90 RUSSIA INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 91 RUSSIA CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 92 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 93 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 95 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 96 SPAIN SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 97 SPAIN BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 98 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 99 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 100 SPAIN INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 101 SPAIN CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 102 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 103 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 104 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 105 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 106 TURKEY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 107 TURKEY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 108 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 109 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 110 TURKEY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 111 TURKEY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 112 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 113 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 114 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 115 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 116 NETHERLANDS SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 117 NETHERLANDS BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 118 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 119 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 120 NETHERLANDS INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 121 NETHERLANDS CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 122 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 123 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 124 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 125 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 126 SWITZERLAND SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 127 SWITZERLAND BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 128 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 129 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 130 SWITZERLAND INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 131 SWITZERLAND CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 132 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 133 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 134 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 135 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 136 BELGIUM SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 137 BELGIUM BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 138 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 139 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 140 BELGIUM INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 141 BELGIUM CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 142 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 143 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 144 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 145 REST OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SEGMENTATION
FIGURE 2 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SEGMENTATION
FIGURE 11 THE WIDE RANGE OF CLINICAL APPLICATION OF INDUCED PLURIPOTENT STEM CELLS (IPSC) ARE EXPECTED TO DRIVE THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SKIN CELLS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
FIGURE 15 PREVALENCE OF CHRONIC DISEASES
FIGURE 16 NUMBER OF PEOPLE WITH DIABETES (MILLION) AMONG AGES 20–79 YEARS
FIGURE 17 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, 2021
FIGURE 18 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, 2020-2029 (USD MILLION)
FIGURE 19 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, CAGR (2022-2029)
FIGURE 20 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, LIFELINE CURVE
FIGURE 21 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, 2021
FIGURE 22 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, 2020-2029 (USD MILLION)
FIGURE 23 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 24 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, 2021
FIGURE 26 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, 2020-2029 (USD MILLION)
FIGURE 27 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 28 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 29 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, 2021
FIGURE 30 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, 2020-2029 (USD MILLION)
FIGURE 31 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 32 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 33 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, 2021
FIGURE 34 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 35 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, CAGR (2022-2029)
FIGURE 36 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 38 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 39 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 40 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SNAPSHOT (2021)
FIGURE 42 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2021)
FIGURE 43 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2022 & 2029)
FIGURE 44 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2021 & 2029)
FIGURE 45 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE (2022-2029)
FIGURE 46 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY SHARE 2021 (%)

منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.