Europe Carrier Screening Market
حجم السوق بالمليار دولار أمريكي
CAGR :
% 
 USD
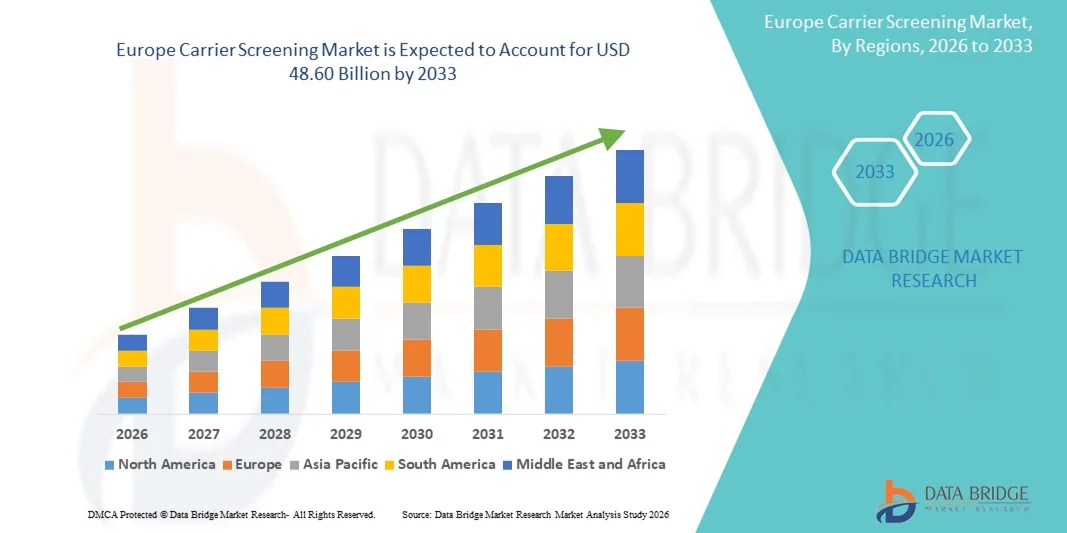
16.92 Billion
USD
48.60 Billion
2025
2033
USD
16.92 Billion
USD
48.60 Billion
2025
2033
| 2026 –2033 | |
| USD 16.92 Billion | |
| USD 48.60 Billion | |
|
|
|
|
تجزئة سوق فحص الناقلات في أوروبا، حسب نوع الاختبار (اختبار الفحص الجزيئي واختبار الفحص الكيميائي الحيوي)، نوع المرض (التليف الكيسي، تاي ساكس، داء غوشيه، فقر الدم المنجلي، ضمور العضلات الشوكي، وغيرها من الاضطرابات الوراثية الجسدية المتنحية)، الحالة الطبية (أمراض الرئة، أمراض الدم، الأمراض العصبية، وغيرها)، التكنولوجيا (تسلسل الحمض النووي، تفاعل البوليميراز المتسلسل، المصفوفات الدقيقة، وغيرها)، الاستخدام النهائي (المستشفيات، المختبرات المرجعية، عيادات الأطباء، وغيرها) - اتجاهات الصناعة وتوقعاتها حتى عام 2033
حجم سوق فحص الناقل في أوروبا
- تم تقييم حجم سوق فحص الناقل في أوروبا بـ 16.92 مليار دولار أمريكي في عام 2025 ومن المتوقع أن يصل إلى 48.60 مليار دولار أمريكي بحلول عام 2033 ، بمعدل نمو سنوي مركب قدره 14.00٪ خلال الفترة المتوقعة
- يُعزى نمو السوق بشكل كبير إلى تزايد الوعي بالاضطرابات الوراثية والطلب المتزايد على الكشف المبكر عن حاملي المرض بين الآباء المحتملين. تُمكّن التطورات في تقنيات الفحص الجزيئي والكيميائي الحيوي، إلى جانب توافر مجموعات حاملي المرض الموسعة، من إجراء اختبارات أكثر دقة وشمولاً وسرعة، مما يُعزز اعتمادها في المستشفيات والعيادات ومختبرات التشخيص.
- علاوة على ذلك، فإن تفضيل المستهلكين المتزايد للرعاية الصحية الشخصية وتخطيط الإنجاب، إلى جانب سهولة إجراء حلول فحص الناقلين في المنزل وفي المختبرات، يُرسّخ فحص الناقلين كممارسة أساسية في رعاية ما قبل الولادة وما قبل الحمل. تُسرّع هذه العوامل المتقاربة من الإقبال على خدمات فحص الناقلين، مما يُعزز توسع السوق بشكل كبير.
تحليل سوق فحص الناقل في أوروبا
- إن فحص الناقل، الذي يحدد الأفراد الذين يحملون طفرات جينية للاضطرابات الجسدية المتنحية والمرتبطة بالكروموسوم X، أصبح بشكل متزايد مكونًا حيويًا للرعاية الصحية الإنجابية الوقائية في كل من البيئات السريرية والمنزلية نظرًا لقدرته على توجيه قرارات تخطيط الأسرة المستنيرة والحد من خطر انتقال الأمراض الوراثية.
- إن الطلب المتزايد على فحص الناقل مدفوع في المقام الأول بالتقدم التكنولوجي مثل تسلسل الجيل التالي، والاعتماد المتزايد على اللجان الموسعة والشاملة للأعراق، والمبادرات الحكومية والرعاية الصحية المتزايدة التي تروج للاختبارات الجينية، والوعي المتزايد بين المستهلكين ومقدمي الرعاية الصحية حول أهمية الكشف المبكر والرعاية الوقائية.
- سيطرت ألمانيا على سوق فحص الناقل بسبب بنيتها التحتية القوية للرعاية الصحية وقدراتها التشخيصية المتقدمة والاعتماد الكبير على الاختبارات الجينية في رعاية ما قبل الولادة وما قبل الحمل.
- من المتوقع أن تكون المملكة المتحدة أسرع دولة نموًا في سوق فحص الناقل خلال فترة التنبؤ بسبب زيادة الوعي بالاضطرابات الوراثية وتوسيع برامج الاختبار قبل الولادة وقبل الحمل واعتماد تقنيات الفحص من الجيل التالي.
- هيمنت اختبارات الفحص الجزيئي على السوق بحصة سوقية بلغت 62.8%، بفضل دقتها العالية في الكشف عن الطفرات الجينية، واعتمادها المتزايد بين الآباء والأمهات الراغبين في الكشف المبكر عن الاضطرابات الوراثية. تُفضّل الاختبارات الجزيئية لقدرتها على تحديد حاملي حالات متعددة في اختبار واحد، مما يوفر نتائج شاملة ويقلل الحاجة إلى تكرار الاختبارات. يوصي مقدمو الرعاية الصحية بشدة بالفحص الجزيئي نظرًا لموثوقيته وفعاليته السريرية في مختلف الفئات السكانية. كما أن تزايد توفر لوحات تسلسل الجيل التالي وتحليلات الطفرات المستهدفة يعزز هيمنة الاختبارات الجزيئية في الممارسة السريرية.
نطاق التقرير وتجزئة سوق فحص الناقل
|
صفات |
رؤى السوق الرئيسية لفحص الناقل |
|
القطاعات المغطاة |
|
|
الدول المغطاة |
أوروبا
|
|
اللاعبون الرئيسيون في السوق |
|
|
فرص السوق |
|
|
مجموعات معلومات البيانات ذات القيمة المضافة |
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم إعدادها بواسطة Data Bridge Market Research تتضمن أيضًا تحليلًا متعمقًا من الخبراء وعلم الأوبئة للمرضى وتحليل خطوط الأنابيب وتحليل التسعير والإطار التنظيمي. |
اتجاهات سوق فحص الناقل في أوروبا
تزايد استخدام لوحات فحص الناقل الموسعة والمنزلية
- يشهد سوق فحص حاملي الجينات توجهًا متزايدًا نحو توسيع نطاق الفحوصات المنزلية، مدفوعةً بتزايد الوعي بالاضطرابات الوراثية والحاجة إلى رعاية صحية إنجابية استباقية. توفر هذه الفحوصات اختبارات شاملة يمكنها الكشف عن العديد من الحالات الوراثية، مما يُمكّن من اتخاذ قرارات تخطيط أسري مدروسة.
- على سبيل المثال، تُقدم مجموعات الاختبار المنزلية "Peaches & Me" و"23 Pears" من Mitera فحصًا منزليًا متعدد الحالات، مما يُحسّن إمكانية الوصول للمستهلكين ويشجع على تبنيها مبكرًا. تُقلل هذه الحلول المنزلية من الحاجة إلى الزيارات السريرية، وتوفر خيارات مُريحة للفئات المُلِمّة بالتكنولوجيا والمهتمة بالصحة.
- تُحسّن التقنيات الجزيئية والكيميائية الحيوية المتقدمة دقة الاختبارات وتُقلل من وقت الاستجابة، مما يسمح بنتائج أكثر موثوقية. كما تدعم هذه التحسينات التكنولوجية تقييم المخاطر الشخصي، مما يُمكّن مُقدمي الرعاية الصحية من تقديم استشارات مُستهدفة بناءً على نتائج الاختبارات.
- يدعم التوافر المتزايد للوحات فحص حاملي الأمراض الوراثية الخاصة بالسكان والجماعات العرقية المختلفة اعتمادها عبر مجموعات سكانية متنوعة. بالإضافة إلى ذلك، تضمن هذه اللوحات الكشف الأوسع عن الحالات الوراثية النادرة، مما يجعلها ضرورية بشكل متزايد لإدارة الصحة الإنجابية الشاملة.
- يُحسّن دمج التقارير الرقمية والاستشارات الجينية عن بُعد مع خدمات فحص الناقل تجربة المستخدم. تتيح هذه الابتكارات للمرضى الحصول على نتائج الفحوصات بأمان عبر الإنترنت والحصول على إرشادات الخبراء، مما يعزز ثقة المستهلك في الفحوصات.
- يُشكّل مزيج التقدم التكنولوجي والراحة وسهولة الوصول سوقًا، ويُرسّخ مكانة الأجهزة الموسعة والمنزلية كعنصر أساسي في الرعاية الصحية الإنجابية الوقائية. ومن المتوقع أن يُحافظ هذا التوجه على النمو في القطاعات السريرية، وخدمات المستهلكين، والرعاية الصحية عن بُعد.
ديناميكيات سوق فحص الناقل في أوروبا
سائق
تزايد الوعي بالاضطرابات الوراثية
- يُعدّ ازدياد الوعي بالاضطرابات الوراثية بين الآباء المحتملين ومقدمي الرعاية الصحية وصانعي السياسات محركًا رئيسيًا لسوق فحص الناقلين. ويشجع الفهم الأعمق للمخاطر الوراثية على إجراء الفحوصات المبكرة واتخاذ قرارات إنجابية مدروسة.
- على سبيل المثال، أطلقت شركات مثل ناتيرا وفولجنت جينيتكس مجموعات موسعة من حاملي المرض وحملات توعية لتثقيف المستهلكين حول فوائد الكشف المبكر. بالإضافة إلى ذلك، تعزز هذه المبادرات تبني هذه التقنية في المستشفيات والعيادات وخدمات الفحص المنزلي، مما يزيد من انتشارها في السوق.
- يدفع التركيز المتزايد على الرعاية الصحية الوقائية والطب الشخصي أنظمة الرعاية الصحية إلى دمج فحص حاملي الجينات في برامج الرعاية الروتينية قبل الحمل وقبل الولادة. يُعزز هذا التكامل الكشف المبكر عن عوامل الخطر ويُقلل من احتمالية إصابة الأبناء بالاضطرابات الوراثية.
- تُحسّن المبادرات التعليمية، والتواصل عبر وسائل التواصل الاجتماعي، وخدمات الاستشارة الوراثية فهمَ وقبولَ فحص الناقل الجيني. كما تُمكّن هذه الجهود الآباءَ المُحتملين من اتخاذ خيارات إنجابية واعية، مما يُسرّع نمو السوق.
- لا يزال دعم البرامج الحكومية والمنظمات الخاصة للصحة الإنجابية، إلى جانب التقدم التكنولوجي في مجال الفحص، يدفع عجلة تبني هذا النوع من الخدمات. تضمن هذه الجهود المنسقة توسعًا في الوصول إليها، وترسيخ فحص حاملي الأمراض كجزء أساسي من الرعاية الصحية الإنجابية.
ضبط النفس/التحدي
التكلفة العالية والتغطية التأمينية المحدودة
- تُشكّل التكلفة العالية لاختبارات الفحص الشامل لحاملي الأمراض عائقًا كبيرًا أمام تبنيها في السوق. فهذه التكاليف قد تُقيّد الوصول إلى الخدمات للفئات المُهتمة بالأسعار، وتُقلّل من الإقبال عليها بشكل عام في كلٍّ من القطاعين الطبي والمستهلك.
- على سبيل المثال، لا تزال لوحات التشخيص الموسعة من شركات مثل Invitae وMyriad Genetics باهظة الثمن، مما يحد من إمكانية الوصول إليها ويثبط تبنيها في المناطق ذات الموارد المالية المحدودة. بالإضافة إلى ذلك، يحد هذا من انتشار حلول الاختبارات المتميزة التي توفر تغطية أوسع للأمراض.
- تُفاقم محدودية التغطية التأمينية والتعويضات المالية التحديات المالية، لا سيما في البلدان التي تُدفع فيها نفقات الرعاية الصحية من جيوب الآباء. تُصعّب هذه القيود على العديد من الآباء المُحتملين تحمّل تكاليف خدمات الفحوصات المُتقدّمة.
- في حين أن خيارات الفحص الأساسية أكثر تكلفة، إلا أن اللوحات المتميزة التي توفر دقة أعلى وتغطية أوسع للحالات قد تُثني المستخدمين عن اختيار الفحص. إضافةً إلى ذلك، فإن سياسات التغطية غير المتسقة بين شركات التأمين تُقلل من الوصول الموحد لخدمات فحص شركات التأمين.
- يتطلب التغلب على هذه العوائق بذل جهود لخفض تكاليف الفحص، وتوسيع نطاق التغطية التأمينية، وتوعية المستهلكين بأهمية فحص شركات التأمين. وتُعدّ معالجة هذه القيود المالية وقيود التغطية التأمينية أمرًا أساسيًا لضمان تكافؤ الفرص والنمو المستدام في كل من الأسواق المتقدمة والناشئة.
نطاق سوق فحص الناقل في أوروبا
يتم تقسيم السوق على أساس نوع الاختبار ونوع المرض والحالة الطبية والتكنولوجيا والاستخدام النهائي.
- حسب نوع الاختبار
بناءً على نوع الاختبار، يُقسّم سوق فحص حاملي الجينات إلى اختبارات فحص جزيئي واختبارات فحص كيميائي حيوي. هيمنت اختبارات الفحص الجزيئي على السوق محققةً أكبر حصة من إيرادات السوق بنسبة 62.8% في عام 2025، بفضل دقتها العالية في الكشف عن الطفرات الجينية واعتمادها المتزايد بين الآباء والأمهات الراغبين في الكشف المبكر عن الاضطرابات الوراثية. تُفضّل الاختبارات الجزيئية لقدرتها على تحديد حاملي حالات متعددة في اختبار واحد، مما يوفر نتائج شاملة ويقلل الحاجة إلى تكرار الاختبارات. يُوصي مُقدّمو الرعاية الصحية بشدة بالفحص الجزيئي نظرًا لموثوقيته وفعاليته السريرية في مختلف الفئات السكانية. كما أن التوافر المتزايد للوحات تسلسل الجيل التالي وتحليلات الطفرات المُستهدفة يُعزز هيمنة الاختبارات الجزيئية في الممارسة السريرية.
من المتوقع أن يشهد قطاع اختبارات الفحص الكيميائي الحيوي أسرع معدل نمو بنسبة 19.4% بين عامي 2026 و2033، مدفوعًا بالبحوث الجارية حول أساليب الفحص السريعة والفعّالة من حيث التكلفة. على سبيل المثال، تعمل شركات مثل Natera وInvitae على توسيع نطاق فرق الفحص الكيميائي الحيوي لتشمل تغطية أوسع للأمراض، مما يوفر عمليات جمع عينات أبسط وأوقات استجابة أسرع. يزداد اعتماد الاختبارات الكيميائية الحيوية في المناطق ذات الوصول المحدود إلى المختبرات الجزيئية المتقدمة نظرًا لقلة متطلبات البنية التحتية. كما أن تزايد الوعي بين الأطباء والمرضى بالكشف المبكر والرعاية الوقائية يدعم اعتماد الاختبارات الكيميائية الحيوية.
- حسب نوع المرض
بناءً على نوع المرض، يُقسّم سوق فحص حاملي الجين إلى التليف الكيسي، وداء تاي ساكس، وداء غوشيه، وداء فقر الدم المنجلي، وضمور العضلات الشوكي، وغيرها من الاضطرابات الوراثية الجسدية المتنحية. وسيُهيمن قطاع التليف الكيسي على السوق بحصة سوقية تبلغ 28.5% بحلول عام 2025، وذلك بفضل الانتشار الواسع لحاملي التليف الكيسي في العديد من الفئات السكانية، والإرشادات المُعتمدة التي تُوصي بالفحص الروتيني. يُوفر فحص التليف الكيسي معلومات بالغة الأهمية لتنظيم الأسرة، ويُقلل من خطر انتقال الاضطرابات الوراثية الشديدة. وقد استثمرت شبكات المختبرات والعيادات بشكل كبير في مجموعات تشمل طفرات التليف الكيسي، مما عزز ريادتها في السوق.
من المتوقع أن يشهد قطاع ضمور العضلات الشوكي أسرع معدل نمو سنوي مركب بنسبة 18.7% بين عامي 2026 و2033، مدفوعًا بتزايد حالات ضمور العضلات الشوكي والتطورات في تقنيات التشخيص المبكر. على سبيل المثال، تُقدم شركات مثل فولجنت جينيتكس وبلوبرينت جينيتكس مجموعات موسعة من حاملي المرض، بما في ذلك الكشف عن ضمور العضلات الشوكي، مما يعزز استراتيجيات التدخل المبكر. كما أن تزايد الوعي بعلاجات ضمور العضلات الشوكي وبرامج فحص حديثي الولادة يشجع على تبني هذه الخدمات. ويتزايد تركيز مقدمي الرعاية الصحية على فحص ضمور العضلات الشوكي نظرًا لتأثيره على نتائج العلاج المبكر وجودة الحياة.
- حسب الحالة الطبية
بناءً على الحالة الطبية، يُقسّم سوق فحص حاملي الجينات إلى أمراض الرئة، وأمراض الدم، والأمراض العصبية، وغيرها. وسيُهيمن قطاع أمراض الرئة على السوق محققًا أكبر حصة من إيرادات السوق بنسبة 31.2% بحلول عام 2025، ويعود ذلك أساسًا إلى انتشار أمراض مثل التليف الكيسي واضطرابات الرئة الوراثية الأخرى. يُعد فحص أمراض الرئة أمرًا بالغ الأهمية للتشخيص المبكر، وتحديد حاملي الجينات، وتقديم الاستشارات الشخصية. وقد طورت المختبرات مجموعات شاملة تُعنى بأمراض الرئة المتعددة في آنٍ واحد، مما يُعزز اعتماد الاختبارات في مراكز رعاية ما قبل الولادة وما قبل الحمل.
من المتوقع أن يشهد قطاع الأمراض العصبية أسرع معدل نمو بنسبة 20.1% بين عامي 2026 و2033، مدفوعًا بتزايد الوعي بالاضطرابات الوراثية العصبية وظهور تقنيات تشخيصية قادرة على اكتشاف ضمور العضلات الشوكي (SMA) ومتلازمة تاي ساكس والحالات ذات الصلة. على سبيل المثال، تحظى لوحات فحص الاضطرابات العصبية من شركة Invitae باعتماد متزايد في العيادات والمستشفيات نظرًا لقدرتها على الكشف المبكر عن حاملي المرض. ومن المتوقع أن يؤدي التقدم في أبحاث علم الوراثة العصبية وتوسيع نطاق التغطية التأمينية للاختبارات الجينية إلى زيادة الطلب. ويوصي الأطباء بشكل متزايد بإجراء اختبارات الأمراض العصبية للفئات المعرضة للخطر، مما يُسرّع نمو السوق.
- حسب التكنولوجيا
بناءً على التكنولوجيا، يُقسّم سوق فحص حاملي الجينات إلى تسلسل الحمض النووي، وتفاعل البوليميراز المتسلسل (PCR)، والمصفوفات الدقيقة، وغيرها. وقد هيمن قطاع تسلسل الحمض النووي على السوق محققًا أكبر حصة من إيرادات السوق بنسبة 55.6% بحلول عام 2025، بفضل دقته العالية، وقابليته للتوسع، وقدرته على اكتشاف طيف واسع من الطفرات عبر جينات متعددة في آنٍ واحد. ويُفضّل تسلسل الحمض النووي على نطاق واسع لفحص حاملي الجينات قبل الولادة وقبل الحمل، مما يتيح رؤى شاملة ونتائج استشارية أفضل. وقد عزز اعتماد تسلسل الجيل التالي (NGS) كفاءة وفعالية تكلفة اختبارات حاملي الجينات القائمة على الحمض النووي.
من المتوقع أن يشهد قطاع تفاعل البوليميراز المتسلسل (PCR) أسرع معدل نمو سنوي مركب بنسبة 19.2% بين عامي 2026 و2033، بفضل قدرته على الكشف السريع، وفعاليته من حيث التكلفة، وإمكانية تطبيقه في تحليل الطفرات المستهدفة. على سبيل المثال، تعمل شركات مثل GeneDx وMyriad Genetics على توسيع نطاق عروض الفحص القائمة على تفاعل البوليميراز المتسلسل للكشف السريع عن الطفرات عالية الانتشار. ولا تزال تقنية تفاعل البوليميراز المتسلسل تحظى باعتماد واسع في المختبرات الصغيرة والمناطق ذات البنية التحتية المحدودة لـ NGS. كما أن سهولة تطبيق فحوصات تفاعل البوليميراز المتسلسل وحساسيتها العالية تجعلها مناسبة لبرامج الكشف الروتينية عن حاملي الجينات، مما يعزز النمو.
- حسب الاستخدام النهائي
بناءً على الاستخدام النهائي، يُقسّم سوق فحص حاملي الجينات إلى مستشفيات، ومختبرات مرجعية، وعيادات وعيادات طبية، وغيرها. وسيُهيمن قطاع المستشفيات على السوق محققًا أكبر حصة من إيرادات السوق بنسبة 42.3% بحلول عام 2025، مدفوعًا بدمج فحص حاملي الجينات في خدمات رعاية ما قبل الولادة، والاستشارات قبل الحمل، والفحوصات الجينية. وتستفيد المستشفيات من قدرات المختبرات الداخلية وقدرتها على توفير مسارات رعاية متكاملة للمرضى، مما يُعزز تبني هذه الخدمات. وتتعاون شبكات المستشفيات الكبيرة والمراكز الطبية الأكاديمية بشكل متزايد مع شركات التشخيص لتقديم مجموعات موسعة من فحص حاملي الجينات، مما يضمن رعاية شاملة للمرضى.
من المتوقع أن يشهد قطاع المختبرات المرجعية أسرع معدل نمو بنسبة 21.5% بين عامي 2026 و2033، مدفوعًا بتوسع خدمات الاختبارات الجينية عالية الإنتاجية. على سبيل المثال، تستثمر مختبرات مثل Quest Diagnostics وLabcorp في منصات فحص متقدمة لناقلات الأمراض لخدمة العديد من المستشفيات والعيادات بكفاءة. كما تستفيد المختبرات المرجعية من الأتمتة والاختبارات المتعددة لتحسين أوقات الاستجابة والدقة. إن القدرة على توفير حلول اختبار فعالة من حيث التكلفة وواسعة النطاق تضع المختبرات المرجعية كقطاع سريع النمو في سوق فحص ناقلات الأمراض.
تحليل إقليمي لسوق فحص الناقل في أوروبا
- سيطرت ألمانيا على سوق فحص الناقل بأكبر حصة من الإيرادات في عام 2025، مدفوعة ببنيتها التحتية القوية للرعاية الصحية، وقدراتها التشخيصية المتقدمة، والاعتماد الكبير على الاختبارات الجينية في رعاية ما قبل الولادة وما قبل الحمل.
- أدى تركيز الدولة على الرعاية الصحية الوقائية، إلى جانب الوعي الواسع بالاضطرابات الوراثية، إلى تسريع استخدام خدمات فحص حاملي الأمراض. كما أن الحضور القوي لشركات التشخيص الرائدة، والبحث والتطوير المستمر في تقنيات الفحص الجزيئي والكيميائي الحيوي، والتعاون مع المستشفيات ومؤسسات البحث، يعزز توسع السوق.
- إن التركيز الألماني على دمج فحص الناقل في البروتوكولات السريرية القياسية، وتحسين استشارة المرضى، ودعم الرعاية الصحية الشخصية يعزز مكانتها القيادية في السوق الإقليمية
نظرة عامة على سوق فحص شركات النقل في المملكة المتحدة
من المتوقع أن يُسجل سوق المملكة المتحدة أسرع معدل نمو سنوي مركب في سوق فحص حاملي الجينات في أوروبا خلال الفترة 2026-2033، مدفوعًا بتزايد الوعي بالاضطرابات الوراثية، وتوسيع برامج فحص ما قبل الولادة وما قبل الحمل، واعتماد تقنيات الفحص من الجيل التالي. وتساهم المبادرات الحكومية والصحية المتزايدة التي تُشجع على الكشف المبكر، إلى جانب الشراكات الاستراتيجية بين المختبرات المحلية وشركات التشخيص الدولية، في زيادة الطلب في السوق. على سبيل المثال، تتعاون شركات مثل Natera وInvitae مع المستشفيات التابعة لهيئة الخدمات الصحية الوطنية (NHS) لتوسيع نطاق الوصول إلى فحص حاملي الجينات. ويساهم تركيز المملكة المتحدة على تحسين نتائج المرضى، وتعزيز الوصول إلى الاختبارات الجينية المتقدمة، ودعم الرعاية الوقائية، في جعلها أسرع الأسواق نموًا في المنطقة.
نظرة عامة على سوق فحص شركات النقل في فرنسا
من المتوقع أن تشهد فرنسا نموًا مطردًا بين عامي 2026 و2033، مدعومًا بتوسع خدمات الرعاية الصحية، وعيادات الخصوبة، وبرامج رعاية ما قبل الولادة، بالإضافة إلى تزايد اعتماد لجان الفحص الشامل لحاملي الجينات. ويعزز تركيز الدولة على التشخيص المبكر للاضطرابات الوراثية، والرعاية الصحية الوقائية، وتكامل التقنيات الجزيئية، الطلب على خدمات فحص حاملي الجينات التي توفر الدقة، وتغطية واسعة للأمراض، ونتائج استشارية موثوقة. كما أن زيادة الاستثمارات في مختبرات التشخيص المتقدمة، إلى جانب الدعم الحكومي القوي لبرامج الصحة الإنجابية، تُحسّن معدلات اعتماد هذه الخدمات في العيادات والمستشفيات المحلية. ويعزز التعاون بين مقدمي خدمات التشخيص الفرنسيين والشركات العالمية تكامل التكنولوجيا وجودة الخدمات. ويعزز التزام فرنسا بتطوير الرعاية الوقائية والكفاءة التشغيلية توقعاتها المستقرة لسوقها في المنطقة الأوروبية.
حصة سوق فحص الناقل في أوروبا
وتدار صناعة فحص الناقل بشكل أساسي من قبل شركات راسخة، بما في ذلك:
- يوروفينس العلمية (لوكسمبورغ)
- شركة إنفيتاي (الولايات المتحدة)
- شركة أوبكو هيلث (الولايات المتحدة)
- شركة لومينكس (الولايات المتحدة)
- فولجنت جينيتكس (الولايات المتحدة)
- تشخيصات كويست (الولايات المتحدة)
- شركة سيما 4 أوبكو، المحدودة (الولايات المتحدة)
- ميرياد جينيتكس (الولايات المتحدة)
- شركة إيلومينا (الولايات المتحدة)
- شركة ثيرمو فيشر العلمية (الولايات المتحدة)
- ميدجينوم (الولايات المتحدة)
- شركة ميرياد جينيتكس (الولايات المتحدة)
- شركة ناتيرا (الولايات المتحدة)
- جين باي جين المحدودة (الولايات المتحدة)
- شركة مختبرات أمريكا القابضة (الولايات المتحدة)
- شركة جبل سيناء للجينوميات (الولايات المتحدة)
- شركة أوتوجينتكس (الولايات المتحدة)
أحدث التطورات في سوق فحص الناقل في أوروبا
- في أكتوبر 2024، أعلنت شركة Myriad Genetics عن إطلاق نظام Foresight Plus لفحص حاملي الجينات، والذي يغطي أكثر من 500 جين. يتيح هذا النظام الموسع كشفًا أشمل للحالات الوراثية النادرة والأقل شيوعًا، مما يُمكّن الأطباء والآباء المحتملين من اكتساب فهم أعمق للمخاطر الوراثية المحتملة. يُعزز هذا الإطلاق التوجه نحو حلول فحص أوسع نطاقًا وتغطية واسعة، ويزيد من الفائدة السريرية لاختبار حاملي الجينات، ويشجع على اعتماده في المستشفيات وعيادات الخصوبة ومراكز التشخيص المتخصصة.
- في يناير 2024، استحوذت ناتيرا على محفظة إنفيتاي للصحة الإنجابية، بما في ذلك خدمات فحص حاملي الجينات وخدمات الاختبارات غير الباضعة قبل الولادة، مقابل 52.5 مليون دولار أمريكي. يعزز هذا الاستحواذ الاستراتيجي مكانة ناتيرا في سوق علم الوراثة الإنجابية، مما يسمح لها بتقديم حلول فحص متكاملة وشاملة. من المتوقع أن تُبسط هذه الخطوة سير العمل السريري، وتُعزز الوصول إلى اختبارات حاملي الجينات المتقدمة، وتدعم تطبيقها على نطاق أوسع بين مقدمي الرعاية الصحية والمرضى الذين يسعون إلى تقييم شامل لمخاطر الإنجاب.
- في فبراير 2023، أطلقت شركة فولجنت جينيتكس نظام Beacon787 الموسّع لفحص حاملي الجينات، والذي يفحص 787 جينًا مرتبطًا بالاضطرابات الجسدية المتنحية والمرتبطة بالكروموسوم X. يُوسّع هذا التطور نطاق فحص حاملي الجينات بشكل كبير، مما يُتيح للآباء والأمهات المحتملين صورةً أكثر شمولًا لملف المخاطر الجينية لديهم. كما يُعزز هذا النظام توجه السوق نحو لوحات شاملة لجميع الأعراق وتغطية واسعة، ويشجع على توسيع نطاق استخدام فحص حاملي الجينات في برامج رعاية ما قبل الولادة وما قبل الحمل في مختلف البيئات السريرية والمخبرية.
- في يناير 2022، أعلنت شركة ميتيرا عن توفر مجموعتي "Peaches & Me" و"23 Pears" لاختبارات الجينات الإنجابية المنزلية في جميع الولايات الأمريكية الخمسين. تتيح هذه المجموعات للمستخدمين فحص حالات مثل متلازمة داون من راحة منازلهم، مما يزيد من سهولة الوصول إليها وراحتها. يُسلط هذا التطور الضوء على الشريحة المتنامية من سوق فحص ناقلات الأمراض التي تركز على المستهلك، ومن المتوقع أن يعزز اعتماد هذه المجموعة بين الفئات المهتمة بالتكنولوجيا والصحية، والتي تسعى إلى تقييم مبكر وسهل لمخاطر الإنجاب.
- في يونيو 2021، أطلقت شركة "جرايل" اختبار الدم "غاليري" متعدد الأورام السرطانية، المصمم لفحص البالغين الذين تزيد أعمارهم عن 50 عامًا أو المعرضين لخطر الإصابة بالسرطان. وبينما يركز هذا الإطلاق بشكل أساسي على الكشف عن السرطان، فإنه يُبرز التوجه الأوسع نحو الاختبارات الجينية متعددة الأمراض وتقنيات الفحص المتقدمة في مجال الرعاية الصحية الوقائية. ويُظهر تطور السوق نحو حلول الكشف المبكر غير الجراحي ودمج أساليب الاختبارات الجينية المبتكرة في الرعاية السريرية الروتينية.
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.