يعرض سوق أجهزة مكافحة الشخير وجراحة الشخير مجموعة من أنواع المنتجات بما في ذلك الأجهزة الفموية، وأجهزة الأنف، وأجهزة التحكم في الوضع، وأشرطة الذقن، وأجهزة علاج EPAP، وأجهزة CPAP. تشكل أجهزة تقدم الفك السفلي (MAD)، وأجهزة تثبيت اللسان (TSD)، وغيرها من الحلول المبتكرة أنواع الأجهزة المتنوعة. وتؤكد هذه المجموعة التزام السوق بمعالجة الشخير من خلال آليات متنوعة، مما يوفر للمستخدمين مجموعة من الخيارات الفعالة المصممة خصيصًا لتلبية الاحتياجات الفردية، مما يبشر بعصر جديد في الرعاية الصحية المتعلقة بالنوم.
الوصول إلى التقرير الكامل @ https://www.databridgemarketresearch.com/reports/north-america-anti-snoring-devices-and-snoring-surgery-market
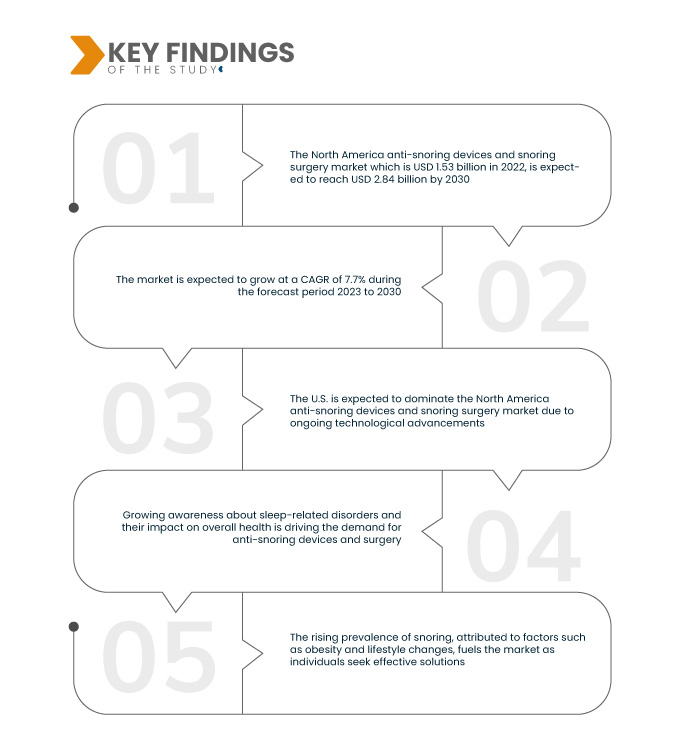
تحلل أبحاث سوق جسر البيانات أن سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية وهو 1.53 مليار دولار أمريكي في عام 2022، ومن المتوقع أن يصل إلى 2.84 مليار دولار أمريكي بحلول عام 2030، بمعدل نمو سنوي مركب قدره 7.7٪ خلال الفترة المتوقعة من 2023 إلى 2030. وتؤدي الاستثمارات المرتفعة في البحث والتطوير إلى دفع سوق أجهزة مكافحة الشخير وجراحة الشخير من خلال تعزيز خلق حلول متقدمة. ويؤدي هذا الالتزام إلى الإدخال المستمر لتقنيات مبتكرة لمكافحة الشخير، وتوسيع السوق، وتلبية احتياجات المستهلكين المتطورة لعلاجات فعالة ومحسنة.
النتائج الرئيسية للدراسة
ومن المتوقع أن تقود الرعاية الصحية الشخصية معدل نمو السوق
يزدهر سوق أجهزة مكافحة الشخير وجراحة الشخير بفضل الاتجاه نحو الرعاية الصحية الشخصية، مع التركيز على الحلول المخصصة لتلبية الاحتياجات الفردية. ويعترف هذا التحول بالطبيعة المتنوعة لأسباب الشخير وتفضيلاته بين المستهلكين. من خلال تقديم أجهزة مخصصة لمكافحة الشخير وأساليب جراحية، يعزز السوق الفعالية ورضا المرضى. يؤكد هذا المحرك على الالتزام بتلبية المتطلبات الفريدة للأفراد، والمساهمة في نمو السوق وتطوره في تلبية متطلبات الرعاية الصحية المتنوعة.
نطاق التقرير وتقسيم السوق
|
تقرير المقياس
|
تفاصيل
|
|
فترة التنبؤ
|
2023 إلى 2030
|
|
سنة الأساس
|
2022
|
|
سنوات تاريخية
|
2021 (قابل للتخصيص حتى 2015-2020)
|
|
الوحدات الكمية
|
الإيرادات بمليار دولار أمريكي، الأحجام بالوحدات، التسعير بالدولار الأمريكي
|
|
القطاعات المغطاة
|
نوع المنتج (أجهزة الفم/الأبواق، أجهزة الأنف، أجهزة التحكم في الوضع، أشرطة الذقن، أجهزة علاج EPAP وأجهزة الضغط الهوائي الإيجابي المستمر (CPAP)، نوع الجهاز (أجهزة تقدم الفك السفلي (MAD)، جهاز تثبيت اللسان (TSD)، المستمر أجهزة ضغط مجرى الهواء الإيجابي (CPAP)، أخرى)، نوع الجراحة (رأب اللهاة والحنك والبلعوم UPPP، وجراحة استئصال اللوزتين واستئصال اللحمية، وجراحات تقدم الفك العلوي والسفلي واللسان اللساني، ورأب اللهاة الحنكية بمساعدة الليزر، واستئصال الترددات الراديوية، وإجراءات العمود، وإجراءات تصلب الحنك، وتجميل الأنف بالحقن وغيرها )، طريقة الشراء (بدون وصفة طبية والوصفة الطبية)، والجنس (ذكر وأنثى)، والمستخدم النهائي (المستشفيات، والرعاية الصحية المجتمعية، وعيادات النوم، والرعاية الصحية المنزلية وغيرها)، وقناة التوزيع (المناقصات المباشرة، ومبيعات التجزئة والمبيعات عبر الإنترنت)
|
|
البلدان المشمولة
|
الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية.
|
|
تغطية لاعبي السوق
|
Apnea Sciences (الولايات المتحدة)، The Pure Sleep Company (الولايات المتحدة)، KELSO & COMPANY (الولايات المتحدة)، ZQuiet (الولايات المتحدة)، GSK plc (المملكة المتحدة)، MPowrx (الولايات المتحدة)، ZYPPAH (الولايات المتحدة)، LivaNova PLC (المملكة المتحدة)، Koninklijke Philips NV (هولندا)، Fisher & Payker Healthcare Limited (نيوزيلندا)، Tomed GmbH (ألمانيا)، SomnoMed (أستراليا)، Meditas Ltd. (المملكة المتحدة)، AirAvant Medical (الولايات المتحدة)، AccuMED Corp. (الولايات المتحدة)، شركة General Electric ( الولايات المتحدة)، Medtronic (أيرلندا)، ResMed (الولايات المتحدة)، Tomed GmbH (ألمانيا)
|
|
نقاط البيانات التي يغطيها التقرير
|
بالإضافة إلى الأفكار حول سيناريوهات السوق مثل القيمة السوقية، ومعدل النمو، والتجزئة، والتغطية الجغرافية، واللاعبين الرئيسيين، تتضمن تقارير السوق التي تنظمها Data Bridge Market Research أيضًا تحليل الخبراء المتعمق، وعلم أوبئة المرضى، وتحليل خطوط الأنابيب، وتحليل الأسعار، والإطار التنظيمي.
|
تحليل القطاع:
يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية على أساس نوع المنتج ونوع الجراحة ونوع الجهاز وطريقة الشراء والجنس والمستخدم النهائي وقناة التوزيع.
- على أساس نوع المنتج، يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية إلى أجهزة الفم/الأبواق، وأجهزة الأنف، وأجهزة التحكم في الوضع، وأشرطة الذقن، وأجهزة علاج EPAP، وأجهزة ضغط مجرى الهواء الإيجابي المستمر (CPAP).
- على أساس نوع الجراحة، يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية إلى جراحة رأب اللهاة والحنك والبلعوم UPPP، وجراحة استئصال اللوزتين واستئصال اللحمية، وجراحات تقدم الفك العلوي والفك السفلي واللسان اللساني، وجراحة رأب اللهاة والحنك اللساني بمساعدة الليزر، واستئصال الترددات الراديوية، وإجراء الأعمدة، والجراحة الحنكية. إجراءات تصلب، الشخير الحقن وغيرها
- على أساس نوع الجهاز، يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية إلى أجهزة تقدم الفك السفلي (MAD)، وأجهزة تثبيت اللسان (TSD)، الضغط الهوائي الإيجابي المستمر أجهزة (CPAP)، وغيرها
- على أساس طريقة الشراء، يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية إلى منتجات بدون وصفة طبية وأخرى تعتمد على الوصفات الطبية.
- على أساس الجنس، يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية إلى ذكور وإناث.
- على أساس المستخدم النهائي، يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية إلى المستشفيات والرعاية الصحية المجتمعية وعيادات النوم، عناية صحية منزلية و اخرين
- على أساس قناة التوزيع، يتم تقسيم سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية إلى المناقصات المباشرة ومبيعات التجزئة والمبيعات عبر الإنترنت.
اللاعبين الرئيسيين
تعترف أبحاث سوق Data Bridge بالشركات التالية كأجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية، واللاعبين في سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية هم Apnea Sciences (الولايات المتحدة)، وThe Pure Sleep Company (الولايات المتحدة)، وKELSO & COMPANY. (الولايات المتحدة)، ZQuiet (الولايات المتحدة)، GSK plc (المملكة المتحدة)، MPowrx (الولايات المتحدة)، ZYPPAH (الولايات المتحدة)، LivaNova PLC (المملكة المتحدة).
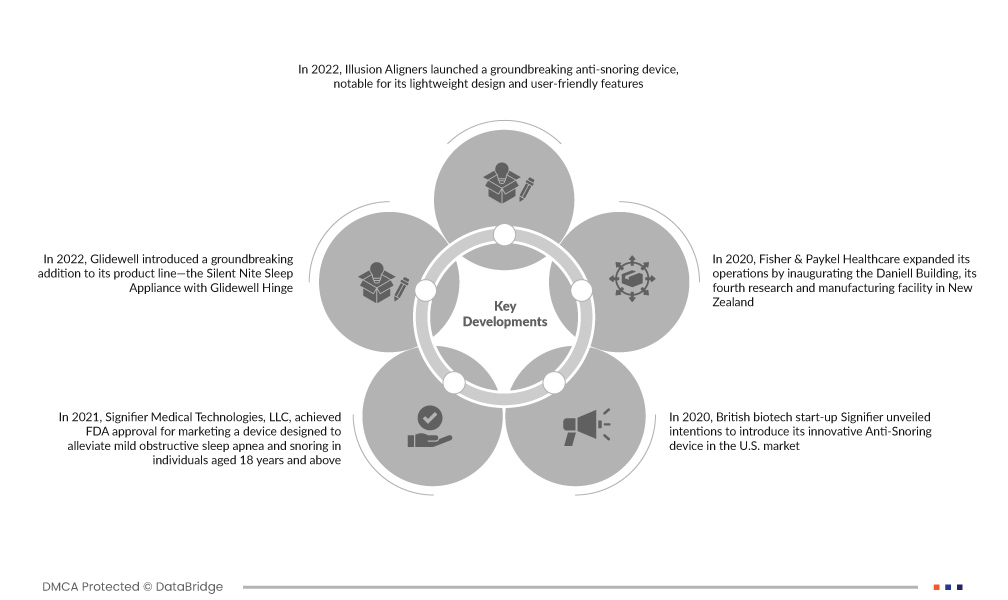
تطورات السوق
- في عام 2022، أطلقت شركة Illusion Aligners جهازًا مبتكرًا لمكافحة الشخير، يتميز بتصميمه خفيف الوزن وميزاته سهلة الاستخدام. لا يعالج هذا الجهاز الفموي بشكل فعال أعراض انقطاع التنفس الانسدادي أثناء النوم (OSA) فحسب، بل يعزز أيضًا جودة النوم بشكل عام، ويقلل من التعب، ويحسن نوعية حياة المستخدم. يمثل ابتكار Illusion Aligners خطوة هامة إلى الأمام في توفير حل بسيط ولكنه مؤثر للقضايا المتعلقة بالنوم
- في عام 2022، قدمت Glidewell إضافة رائدة إلى خط منتجاتها - جهاز Silent Nite Sleep المزود بمفصلة Glidewell. تم تصميم هذا الجهاز المبتكر خصيصًا لمعالجة الشخير وانقطاع التنفس الانسدادي أثناء النوم (OSA)، وهو يعرض تقنية متقدمة لتحسين الفك السفلي. بفضل مفصل Glidewell الفريد، يقدم الجهاز حلاً فعالاً لتحسين جودة النوم وتخفيف أعراض انقطاع التنفس أثناء النوم (OSA)، مما يمثل تقدمًا كبيرًا في علاج اضطرابات النوم.
- في عام 2021، حصلت شركة Signifier Medical Technologies, LLC على موافقة إدارة الغذاء والدواء (FDA) لتسويق جهاز مصمم للتخفيف من انقطاع التنفس الانسدادي الخفيف أثناء النوم والشخير لدى الأفراد الذين تبلغ أعمارهم 18 عامًا فما فوق. يشير هذا الإنجاز التنظيمي إلى سلامة الجهاز وفعاليته، مما يوفر للمرضى حلاً غير جراحي لمعالجة المشكلات المتعلقة بالنوم والمساهمة في تطوير علاجات النوم الفعالة والتي يمكن الوصول إليها.
- في عام 2020، كشفت شركة Signifier البريطانية الناشئة في مجال التكنولوجيا الحيوية عن نيتها تقديم جهازها المبتكر لمكافحة الشخير في السوق الأمريكية. وحددت الشركة هدف تمويل طموح قدره 100 مليون دولار للمشروع. تعكس هذه الخطوة الإستراتيجية التزام Signifier بتلبية الطلب على الحلول المتقدمة لمكافحة الشخير، مما يوضح ثقة الشركة في التأثير المحتمل للجهاز وتفانيها في تأمين دعم كبير لدخول السوق والنمو.
- في عام 2020، قامت شركة Fisher & Paykel Healthcare بتوسيع عملياتها من خلال افتتاح مبنى دانييل، وهو مرفق البحث والتصنيع الرابع التابع لها في نيوزيلندا. ويساهم هذا التطور الاستراتيجي في تمكين الشركة من تعزيز جهودها في البحث وإنتاج حلول الرعاية الصحية المتطورة، لا سيما في مجال اضطرابات النوم. تعكس المنشأة الجديدة التزام شركة Fisher & Paykel Healthcare بالابتكار وتؤكد قدرتها على تقديم منتجات رائدة لتحسين الرعاية الصحية المرتبطة بالنوم.
التحليل الإقليمي
جغرافيًا، البلدان المشمولة في تقرير سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية هي الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية.
وفقًا لتحليل أبحاث سوق Data Bridge:
من المتوقع أن تهيمن الولايات المتحدة على سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية في الفترة المتوقعة 2023-2030
من المتوقع أن تهيمن الولايات المتحدة على سوق أجهزة مكافحة الشخير وجراحة الشخير في أمريكا الشمالية بسبب التقدم التكنولوجي المستمر. يساهم تطوير الأجهزة المبتكرة والقابلة للتخصيص والمصممة لكل من الرجال والنساء في هيمنة البلاد. ويعكس هذا الاتجاه الالتزام بمعالجة المخاوف المتنوعة المتعلقة بالشخير من خلال الحلول المتطورة، مما يضع الولايات المتحدة كلاعب رئيسي في تشكيل مشهد السوق خلال الفترة المتوقعة.
لمزيد من المعلومات التفصيلية حول تقرير سوق أجهزة مكافحة الشخير وجراحات الشخير في أمريكا الشمالية، انقر هنا –https://www.databridgemarketresearch.com/reports/north-america-anti-snoring-devices-and-snoring-surgery-market