يتضمن سوق إعادة معالجة الأجهزة الطبية العالمي تنظيف وتطهير وتعقيم الأجهزة الطبية ذات الاستخدام الواحد لإعادة استخدامها بشكل آمن. تهدف هذه الممارسة المستدامة إلى تقليل تكاليف الرعاية الصحية والنفايات الطبية والأثر البيئي. مع سعي مقدمي الرعاية الصحية إلى إيجاد طرق فعالة لإدارة الموارد، اكتسب سوق إعادة المعالجة أهمية كبيرة. ويشمل مختلف أجهزة طبية، بما في ذلك الأدوات الجراحية، ومعدات التشخيص، وملحقاتها. بشكل عام، يعكس سوق إعادة معالجة الأجهزة الطبية العالمي مشهدًا ديناميكيًا يتشكل من تقاطع العوامل الاقتصادية والبيئية وصناعة الرعاية الصحية.
الوصول إلى التقرير الكامل @ https://www.databridgemarketresearch.com/reports/global-medical-device-reprocessing-market
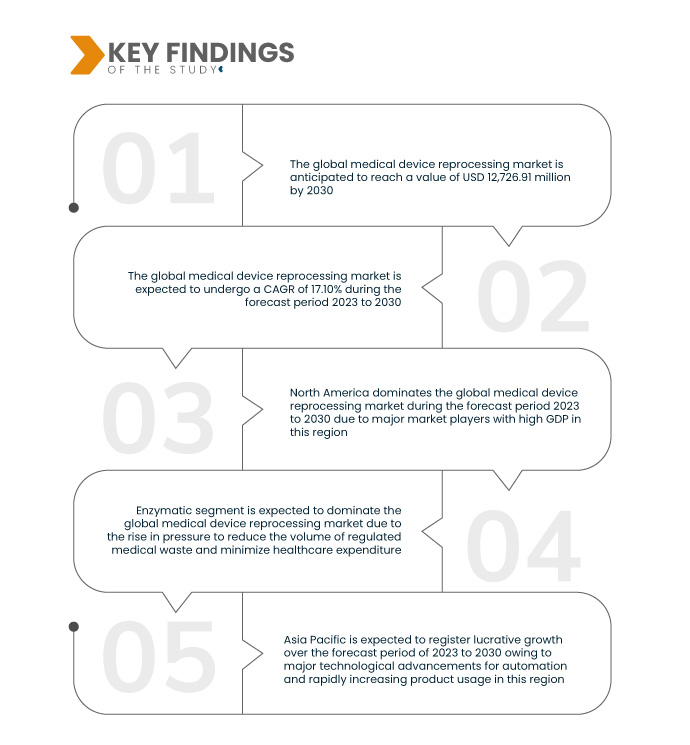
تحلل أبحاث سوق جسر البيانات أن سوق إعادة معالجة الأجهزة الطبية العالمية، والتي كانت 3,599.7 مليون دولار أمريكي في عام 2022، سترتفع إلى 12,726.91 مليون دولار أمريكي بحلول عام 2030 ومن المتوقع أن تشهد معدل نمو سنوي مركب قدره 17.10٪ خلال الفترة المتوقعة من 2023 إلى 2030. وقد لعبت زيادة الوعي والتعليم داخل قطاع الرعاية الصحية دورًا محوريًا في تعزيز قبول إعادة معالجة الأجهزة الطبية. إن المتخصصين في الرعاية الصحية ومؤسساتها، الذين لديهم معرفة أفضل بالفوائد الاقتصادية والبيئية، يدركون بشكل متزايد أن إعادة المعالجة هي ممارسة قيمة ومستدامة لإدارة الرعاية الصحية الفعالة من حيث التكلفة والمسؤولة.
النتائج الرئيسية للدراسة
ومن المتوقع أن يؤدي الضغط لخفض تكاليف الرعاية الصحية إلى دفع معدل نمو السوق
إن التحدي المتصاعد المتمثل في التحكم في نفقات الرعاية الصحية، والذي يتفاقم بسبب تزايد أعداد المرضى والحالات المزمنة، يجبر قطاع الرعاية الصحية على تبني استراتيجيات لتوفير التكاليف. واستجابة لهذا الضغط المالي، اكتسب اعتماد ممارسات مثل إعادة معالجة الأجهزة الطبية أهمية كبيرة. وباعتبارها حلاً قابلاً للتطبيق، تسمح إعادة المعالجة لمقدمي الرعاية الصحية بتقليص النفقات دون المساس بالجودة، بما يتماشى مع الهدف الشامل المتمثل في تحقيق إدارة فعالة ومستدامة للتكاليف في قطاع الرعاية الصحية.
نطاق التقرير وتقسيم السوق
|
تقرير المقياس
|
تفاصيل
|
|
فترة التنبؤ
|
2023 إلى 2030
|
|
سنة الأساس
|
2022
|
|
سنوات تاريخية
|
2021 (قابل للتخصيص حتى 2015-2020)
|
|
الوحدات الكمية
|
الإيرادات بمليون دولار أمريكي، الأحجام بالوحدات، التسعير بالدولار الأمريكي
|
|
القطاعات المغطاة
|
النوع (منظف إنزيمي، غير إنزيمي)، المنتج والخدمة (دعم وخدمات إعادة المعالجة، الأجهزة الطبية المعاد معالجتها)، العملية (النقع المسبق، التنظيف اليدوي، التنظيف التلقائي، التطهير)، نوع الأجهزة (الأجهزة الحرجة، الأجهزة شبه الحرجة، غير الأجهزة الحرجة)، التطبيقات (الأجهزة، الملحقات)، المستخدم النهائي (المستشفيات، العيادات، الرعاية الصحية المنزلية، مراكز التشخيص، الشركات المصنعة، المراكز الجراحية المتنقلة، آحرون)
|
|
البلدان المشمولة
|
الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية وألمانيا وفرنسا والمملكة المتحدة وهولندا وسويسرا وبلجيكا وروسيا وإيطاليا وإسبانيا وتركيا وبقية أوروبا في أوروبا والصين واليابان والهند وكوريا الجنوبية وسنغافورة وماليزيا وأستراليا وتايلاند وإندونيسيا والفلبين وبقية دول آسيا والمحيط الهادئ (APAC) في منطقة آسيا والمحيط الهادئ (APAC) والمملكة العربية السعودية والإمارات العربية المتحدة وجنوب إفريقيا ومصر وإسرائيل وبقية دول الشرق الأوسط وأفريقيا (MEA) كجزء من الشرق الأوسط شرق وأفريقيا (MEA) والبرازيل والأرجنتين وبقية أمريكا الجنوبية كجزء من أمريكا الجنوبية
|
|
تغطية لاعبي السوق
|
Stryker (الولايات المتحدة)، Cardinal Health (الولايات المتحدة)، Johnson & Johnson Private Limited (الولايات المتحدة)، Baxter (الولايات المتحدة)، 3M (الولايات المتحدة)، Cantel Medical (الولايات المتحدة)، STERIS (الولايات المتحدة)، EverX (الولايات المتحدة)، Vanguard AG (الولايات المتحدة). )، Avante Health Solutions (الولايات المتحدة)، SteriPro (كندا)، Getinge AB (السويد)، SureTek Medical (الولايات المتحدة)، SOMA TECH INTL (الولايات المتحدة)، شركة Olympus (اليابان)، Medtronic (أيرلندا)، Pioneer Medical Devices AG (ألمانيا). )
|
|
نقاط البيانات التي يغطيها التقرير
|
بالإضافة إلى الأفكار حول سيناريوهات السوق مثل القيمة السوقية، ومعدل النمو، والتجزئة، والتغطية الجغرافية، واللاعبين الرئيسيين، تتضمن تقارير السوق التي تنظمها Data Bridge Market Research أيضًا تحليل الخبراء المتعمق، وعلم أوبئة المرضى، وتحليل خطوط الأنابيب، وتحليل الأسعار، والإطار التنظيمي.
|
تحليل القطاع:
يتم تصنيف سوق إعادة معالجة الأجهزة الطبية العالمية إلى ستة قطاعات بارزة تعتمد على النوع والمنتج والخدمة والعملية ونوع الأجهزة والتطبيق والمستخدم النهائي.
- على أساس النوع، يتم تقسيم سوق إعادة معالجة الأجهزة الطبية العالمية إلى منظفات إنزيمية وغير إنزيمية. من المتوقع أن يهيمن القطاع الأنزيمي على السوق بحصة سوقية تبلغ 63.51% بسبب ارتفاع الضغط لتقليل حجم النفايات الطبية الخاضعة للرقابة
من المتوقع أن يهيمن القطاع الأنزيمي على سوق إعادة معالجة الأجهزة الطبية
ومن المتوقع أن يهيمن القطاع الأنزيمي على السوق بحصة سوقية تبلغ 63.51% بسبب ارتفاع الضغط لتقليل حجم النفايات الطبية الخاضعة للتنظيم وتقليل نفقات الرعاية الصحية. يثبت النهج الأنزيمي أنه محوري في التوافق مع هذه الأهداف، مما يؤدي إلى ظهوره في سوق إعادة معالجة الأجهزة الطبية.
- على أساس المنتج والخدمة، يتم تقسيم سوق إعادة معالجة الأجهزة الطبية العالمية إلى دعم وخدمات إعادة المعالجة والأجهزة الطبية المعاد معالجتها. من المتوقع أن يهيمن قطاع دعم وخدمات إعادة المعالجة على السوق بحصة سوقية تبلغ 82.70%، حيث تؤدي هذه الأجهزة الطبية المعاد معالجتها إلى وفورات كبيرة في قطاع الرعاية الصحية، وتساعد في تقليل العدوى وتكون بنفس كفاءة الجهاز الأصلي.
- على أساس العملية، يتم تقسيم سوق إعادة معالجة الأجهزة الطبية العالمية إلى النقع المسبق، والتنظيف اليدوي، والتنظيف التلقائي، والتطهير. من المتوقع أن يهيمن قطاع التطهير على السوق بحصة سوقية تبلغ 49.0% بسبب ارتفاع الضغط لتقليل حجم النفايات الطبية الخاضعة للرقابة.
- بناءً على نوع الأجهزة، يتم تقسيم سوق إعادة معالجة الأجهزة الطبية العالمية إلى أجهزة حرجة، وأجهزة شبه حرجة، وأجهزة غير حرجة. ومن المتوقع أن يهيمن قطاع الأجهزة غير الحيوية على السوق بحصة سوقية تبلغ 62.8% بسبب ارتفاع النفايات الطبية وعدوى المستشفيات
من المتوقع أن يهيمن قطاع الأجهزة غير الحرجة على سوق إعادة معالجة الأجهزة الطبية
ومن المتوقع أن يهيمن قطاع الأجهزة غير الحيوية على السوق بحصة سوقية تبلغ 62.8% بسبب ارتفاع النفايات الطبية وعدوى المستشفيات. إن التركيز على مكافحة العدوى والحد من النفايات يدفع إلى تفضيل إعادة معالجة الأجهزة الطبية غير الحرجة، مما يجعلها قطاعًا بارزًا ومحوريًا في السوق.
- على أساس التطبيق، يتم تقسيم سوق إعادة معالجة الأجهزة الطبية العالمية إلى الأجهزة والملحقات. من المتوقع أن يهيمن قطاع الأجهزة على السوق بحصة سوقية تبلغ 78.0% حيث تؤدي هذه الأجهزة إلى وفورات كبيرة في قطاع الرعاية الصحية
- على أساس المستخدم النهائي، يتم تقسيم سوق إعادة معالجة الأجهزة الطبية العالمية إلى المستشفيات والعيادات والرعاية الصحية المنزلية ومراكز التشخيص والشركات المصنعة ومراكز الجراحة المتنقلة وغيرها. ومن المتوقع أن يهيمن قطاع المستشفيات على السوق بحصة سوقية تبلغ 39.3% بسبب زيادة التكيف المفاجئ والطلب على الأجهزة الطبية المعاد معالجتها في المستشفيات.
اللاعبين الرئيسيين
تعترف أبحاث سوق Data Bridge بالشركات التالية باعتبارها الشركات العالمية الرئيسية في سوق إعادة معالجة الأجهزة الطبية العالمية في سوق إعادة معالجة الأجهزة الطبية العالمية وهي Avante Health Solutions (الولايات المتحدة)، SteriPro (كندا)، Getinge AB (السويد)، SureTek Medical (الولايات المتحدة)، SOMA TECH INTL (الولايات المتحدة)، شركة Olympus (اليابان)، Medtronic (أيرلندا)، Pioneer Medical Devices AG (ألمانيا)
تطورات السوق
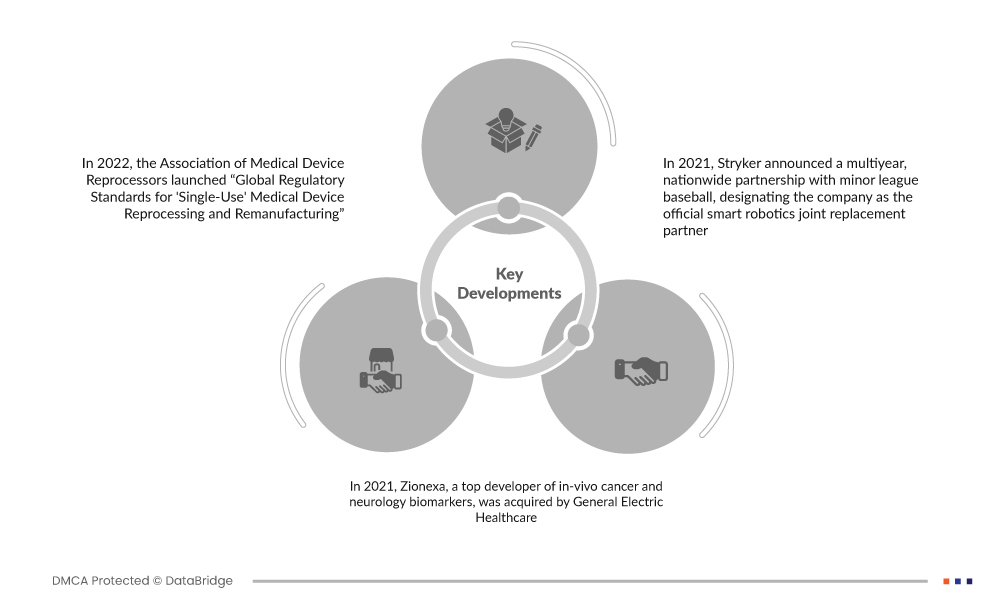
- في عام 2022، أطلقت جمعية إعادة معالجات الأجهزة الطبية "المعايير التنظيمية العالمية لإعادة معالجة وإعادة تصنيع الأجهزة الطبية ذات الاستخدام الواحد". إنها أول خارطة طريق لمساعدة الهيئات المبلغة ووزارات الصحة والسلطات التنظيمية للأجهزة الطبية للوصول إلى فوائدها العالمية للمستشفيات والأنظمة الصحية
- في عام 2021، استحوذت شركة جنرال إلكتريك هيلثكير على شركة Zionexa، وهي شركة رائدة في تطوير المؤشرات الحيوية للسرطان والأعصاب داخل الجسم الحي. ومن خلال عملية الشراء هذه، ستتمكن الشركات من توفير رعاية صحية أكثر تخصيصًا. تعتزم الشركة تسويق المؤشرات الحيوية في خط أنابيب Zionexa بالإضافة إلى وكيل التصوير المقطعي بالإصدار البوزيتروني (PET) المعتمد مؤخرًا من إدارة الغذاء والدواء الأمريكية CriannaTM (fluoroestradiol F-18)، والذي يُستخدم جنبًا إلى جنب مع الخزعة لتحديد الآفات الإيجابية لمستقبلات هرمون الاستروجين (ER) ومساعدة المرضى الذين يعانون من سرطان الثدي النقيلي أو المتكرر في اتخاذ خيارات العلاج
- في عام 2021، أعلنت شركة Stryker عن شراكة وطنية متعددة السنوات مع دوري البيسبول الصغير، حيث عينت الشركة باعتبارها الشريك البديل الرسمي للروبوتات الذكية. كجزء من تفانيها في تعزيز الرعاية الصحية وكجزء من شراكة أكبر مع MiLB، تعد Stryker أيضًا الشريك المقدم لـ "Own the Walk"، وهي أول مبادرة وطنية على الإطلاق تشجع المشجعين من جميع الأعمار على البقاء نشطين من خلال الأنشطة المنتظمة مثل المشي. توفر هذه الشراكة أساسًا متينًا للتنمية والشهرة المستقبلية
التحليل الإقليمي
جغرافيًا، البلدان المشمولة في تقرير سوق إعادة معالجة الأجهزة الطبية العالمية هي الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية وألمانيا وفرنسا والمملكة المتحدة وهولندا وسويسرا وبلجيكا وروسيا وإيطاليا وإسبانيا وتركيا وبقية أوروبا في أوروبا، الصين واليابان والهند وكوريا الجنوبية وسنغافورة وماليزيا وأستراليا وتايلاند وإندونيسيا والفلبين وبقية دول آسيا والمحيط الهادئ (APAC) والمملكة العربية السعودية والإمارات العربية المتحدة وجنوب أفريقيا ومصر وإسرائيل، بقية دول الشرق الأوسط وأفريقيا (MEA) كجزء من الشرق الأوسط وأفريقيا (MEA)، والبرازيل والأرجنتين وبقية أمريكا الجنوبية كجزء من أمريكا الجنوبية
وفقًا لتحليل أبحاث سوق Data Bridge:
أمريكا الشمالية هي المنطقة المهيمنة في سوق إعادة معالجة الأجهزة الطبية العالمية خلال الفترة المتوقعة 2023-2030
تهيمن أمريكا الشمالية على سوق إعادة معالجة الأجهزة الطبية العالمية، مدفوعة باللاعبين الرئيسيين والناتج المحلي الإجمالي القوي. يتم تعزيز ريادة السوق في المنطقة من خلال المشاركة النشطة في إرشادات أجهزة المساعد الرقمي الشخصي (PDA) والمشهد الديناميكي للأجهزة الطبية المعاد معالجتها. تساهم هذه العوامل مجتمعة في تسريع معدل النمو، مما يجعل أمريكا الشمالية مركزًا محوريًا للتقدم والالتزام بمعايير الصناعة المتطورة في قطاع إعادة معالجة الأجهزة الطبية.
تشير التقديرات إلى أن منطقة آسيا والمحيط الهادئ هي المنطقة الأسرع نموًا في العالم سوق إعادة معالجة الأجهزة الطبية العالمية الفترة المتوقعة 2023-2030
ومن المتوقع أن تهيمن منطقة آسيا والمحيط الهادئ على السوق العالمية لإعادة معالجة الأجهزة الطبية، مدفوعة بالتقدم التكنولوجي الكبير في الأتمتة والزيادة السريعة في استخدام المنتجات. ويعزز نمو السوق في المنطقة الوعي المتزايد بالأجهزة الطبية المعاد معالجتها. هذا التقاء الابتكار التكنولوجي وزيادة الوعي يضع منطقة آسيا والمحيط الهادئ كمساهم كبير في توسيع السوق العالمية لإعادة معالجة الأجهزة الطبية.
للحصول على معلومات أكثر تفصيلاً حول سوق إعادة معالجة الأجهزة الطبية العالمية التقرير اضغط هنا –https://www.databridgemarketresearch.com/reports/global-medical-device-reprocessing-market