На рынке поставок для клинических испытаний услуги играют ключевую роль, включая хранение, производство, упаковку и маркировку. Услуги хранения необходимы для надежного размещения исследуемых продуктов при различных температурных условиях, обеспечивая их целостность. Производственные услуги включают производство материалов для клинических испытаний в соответствии со строгими правилами. Услуги упаковки и маркировки обеспечивают безопасное и точное распространение, предоставляя идентификацию лекарственных средств и инструкции по применению. Вместе эти услуги жизненно важны для поддержки клинических испытаний, гарантируя безопасность продукции, прослеживаемость и соблюдение стандартов качества, которые необходимы для успешных испытаний.
Доступ к полному отчету по адресу https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market
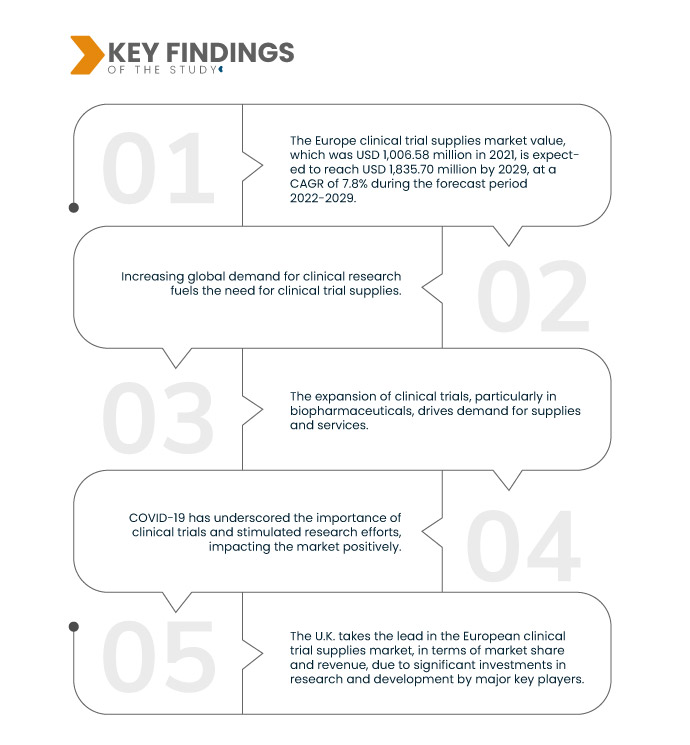
Data Bridge Market Research анализирует, что стоимость европейского рынка поставок для клинических испытаний , которая в 2021 году составила 1 006,58 млн долларов США, как ожидается, достигнет значения 1 835,70 млн долларов США к 2029 году при среднегодовом темпе роста 7,8% в прогнозируемый период 2022-2029 годов. Инновации в технологиях поставок для клинических испытаний стимулируют рынок поставок для клинических испытаний, повышая эффективность и точность. Передовые инструменты и системы оптимизируют управление цепочкой поставок, сокращая ошибки, обеспечивая своевременную доставку и поддерживая успех клинических испытаний.
Основные выводы исследования
Ожидается, что строгое соблюдение нормативных требований будет способствовать темпам роста рынка.
Строгие нормативные требования являются важным фактором на рынке поставок для клинических испытаний. Регулирующие органы требуют точных и соответствующих требованиям поставок для клинических испытаний, чтобы гарантировать безопасность пациентов и целостность данных. Это требует тщательного контроля качества, прослеживаемости и документирования. Рынок реагирует, предлагая специализированные услуги и продукты, которые соответствуют этим строгим стандартам, что способствует росту. Фармацевтические и биотехнологические компании все больше полагаются на соответствующие требованиям поставки для клинических испытаний , чтобы ориентироваться в сложных нормативных условиях и получать одобрения на новые методы лечения.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2022-2029
|
Базовый год
|
2021
|
Исторические годы
|
2020 (Можно настроить на 2014-2019)
|
Количественные единицы
|
Доход в млн. долл. США, объемы в единицах, цены в долл. США
|
Охваченные сегменты
|
Услуги (хранение, производство, упаковка и маркировка), Клиническая фаза (фаза III, фаза II, фаза IV, фаза I), Терапевтическое использование (онкология, сердечно-сосудистые заболевания, дерматология, нарушения обмена веществ, инфекционные заболевания , респираторные заболевания, заболевания центральной нервной системы и психические расстройства, заболевания крови, другие), Конечный пользователь (Контрактные исследовательские организации, фармацевтические и биотехнологические компании)
|
Страны, охваченные
|
Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, остальные страны Европы в Европе.
|
Market Players Covered
|
Movianto (U.S.), Sharp (U.S.),Thermo Fisher Scientific Inc.,(U.S.), Catalent, Inc (U.S.), PCI Pharma Services (U.S.), Almac Group (U.K.), PAREXEL International Corporation (U.S.), Bionical Ltd. (U.K.), Alium Medical Limited (U.K.), Myonex (U.K.), Clinigen Group plc (U.K.), Ancillare, LP (U.S.), SIRO Clinpharm (India) CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC. (U.S.) Biocair (U.K.) and among others.
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The Europe clinical trial supplies market is categorized based on services, clinical phase, therapeutic uses, and end user.
- On the basis of services the Europe clinical trial supplies market is segmented into manufacturing, distribution, storage, and packaging and labelling.
- On the basis of clinical phase the Europe clinical trial supplies market is segmented into phase I, phase II, phase III, and phase IV.
- On the basis of therapeutic uses, the Europe clinical trial supplies market is segmented into oncology, CNS and mental disorders, cardiovascular diseases, infectious disease, respiratory diseases, metabolic disorders, blood disorders, dermatology, and others.
- On the basis of end user, the Europe clinical trial supplies market is segmented into contract research organizations and pharmaceutical and biotechnology companies.
Major Players
Data Bridge Market Research recognizes the following companies as the Europe clinical trial supplies market players in Europe clinical trial supplies market are Movianto (U.S.), Sharp (U.S.),Thermo Fisher Scientific Inc.,(U.S.), Catalent, Inc (U.S.), PCI Pharma Services (U.S.), Almac Group (U.K.), PAREXEL International Corporation (U.S.).
Market Developments
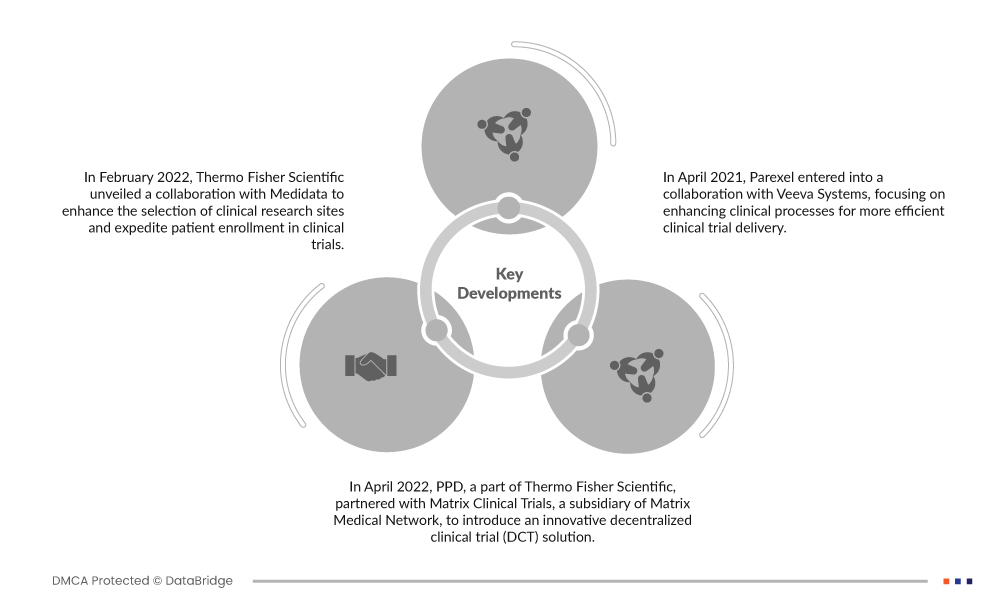
- In February 2022, Thermo Fisher Scientific unveiled a collaboration with Medidata to enhance the selection of clinical research sites and expedite patient enrollment in clinical trials. This collaboration aims to streamline clinical trial planning and execution, ultimately accelerating the process. It leverages extensive datasets from 26,000 clinical trials and nearly 8 million patients conducted in over 140 countries worldwide, ensuring more efficient and effective clinical research.
- In April 2022, PPD, a part of Thermo Fisher Scientific, partnered with Matrix Clinical Trials, a subsidiary of Matrix Medical Network, to introduce an innovative decentralized clinical trial (DCT) solution. This partnership aims to make clinical trials more accessible to patients. The DCT approach allows patients to participate in trials remotely, enhancing convenience and inclusivity while maintaining high-quality data collection and patient safety standards.
- В апреле 2021 года Parexel начал сотрудничество с Veeva Systems, сосредоточившись на улучшении клинических процессов для более эффективной доставки клинических испытаний. Это сотрудничество использует облачную технологию Veeva для оптимизации различных аспектов клинических испытаний с целью ускорения исследований и разработок, улучшения управления данными и улучшения сотрудничества между заинтересованными сторонами в фармацевтической и биотехнологической отраслях.
Региональный анализ
Географически отчет о европейском рынке поставок для клинических исследований охватывает следующие страны: Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция и остальные страны Европы.
Согласно анализу Data Bridge Market Research:
Великобритания будет доминирующим регионом на европейском рынке поставок для клинических исследований в прогнозируемый период 2022–2029 гг.
Великобритания лидирует на европейском рынке поставок для клинических испытаний с точки зрения доли рынка и доходов. Прогнозируется, что это доминирование сохранится в 2022-2029 годах благодаря значительным инвестициям в исследования и разработки со стороны ведущих ключевых игроков. Развитая инфраструктура здравоохранения региона еще больше укрепляет его позиции. Приверженность Великобритании улучшению услуг поставок для клинических испытаний делает ее ключевым игроком на европейском рынке.
Для получения более подробной информации об отчете о рынке поставок для клинических испытаний в Европе нажмите здесь – https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market