U.S. Acute Respiratory Distress Syndrome (ARDS)Market Analysis and Insights
The U.S. acute respiratory distress syndrome (ARDS) market is expected to grow significantly in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 10.6% in the forecast period of 2022 to 2029 and is expected to reach USD 7,083.46 million by 2029. The major factor driving the growth of the market is the Increasing prevalence and incidence of acute lung injury, a wide range of risk factors for ARDS and acceleration in a patient pool of covid-19 with ARDS, the rising rate of air pollution and lifestyle-related diseases, and increasing accident rates and trauma causing ARDS.
Acute respiratory distress syndrome (ARDS) is a life-threatening lung injury that allows fluid to leak into the lungs. Most people who get ARDS are already hospitalized for trauma or illness like COVID-19. The syndrome usually occurs when fluids build up in the lungs' tiny, elastic air sacs called alveoli. This fluid build-up causes less oxygen reaches the bloodstream. This deprives the organs of getting enough oxygen for their normal function. People with other illness develops ARDS within a few hours to days after the precipitating injury or infection. The risk of death increases with age, and depending on the severity of the illness, patients surviving the syndrome becomes hard. Severe illness or injury causing damage to the membrane sacs of the lungs leads to ARDS. The most common underlying causes for the said diseases include sepsis, inhalation of harmful substances, severe pneumonia, head, chest, or another major injury, coronavirus disease 2019 (COVID-19), and others.
The U.S. acute respiratory distress syndrome (ARDS) market report provides details of market share, new developments, and the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, products approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an Analyst Brief. Our team will help you create a revenue impact solution to achieve your desired goal.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Cause (Coronavirus Disease 2019 (COVID-19), Sepsis, Inhalation Of Harmful Substances, Severe Pneumonia And Others), Type (Diagnosis And Treatment), Route Of Administration (Oral, Parenteral And Others), End User (Hospitals, Specialty Clinics, Home Healthcare And Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy And Others) |
|
Countries Covered |
U.S. |
|
Market Players Covered |
Gilead Sciences, Inc., Terumo Medical Corporation, Getinge AB., Medtronic, LivaNova PLC, Fresenius SE & Co. KGaA, Drägerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd, Fisher & Paykel Healthcare Limited., Hamilton Medical, NIPRO, Pfizer Inc., ResMed, Smiths Medical, WEINMANN Emergency Medical Technology GmbH + Co. KG |
Market Definition
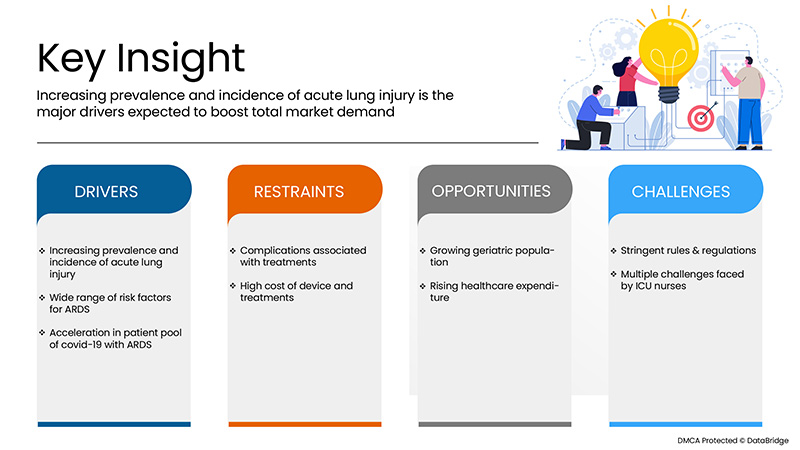
The increasing prevalence and incidence of acute lung injury is an important driver for the U.S. acute respiratory distress syndrome (ARDS) market. A wide range of risk factors for ARDS and acceleration in a patient pool of covid-19 with ARDS accelerate market growth. The major restraints may negatively impact the U.S. acute respiratory distress syndrome (ARDS) market. Complications associated with treatments and high cost of device and treatments. The growing geriatric population is expected to provide market opportunities. However, stringent rules and regulations are projected to challenge U.S. acute respiratory distress syndrome (ARDS) market growth.
U.S. Acute Respiratory Distress Syndrome (ARDS) Market Dynamics
Drivers
- Increasing prevalence and incidence of acute lung injury
Patients with acute lung injuries are being widely reported due to numerous factors like the increasing aged population and the rising number of patients with sepsis and pneumonia, among others. However, most people get diagnosed with lung injuries and acute respiratory distress syndrome only at the late stages. Thereby, the incidence and prevalence of ARDS keep increasing, and the disease has been widely recognized as a major clinical problem worldwide, carrying high morbidity and mortality burden. Hence, increasing prevalence and incidence rates of acute lung injuries and accompanied acute respiratory distress syndrome drive the U.S. acute respiratory distress syndrome (ARDS) market.
- Wide range of risk factors for ARDS
An enormous range of risk factors is reported for acute respiratory distress syndrome. There are environmental and individual risk factors involved with the syndrome. Some chronic alcohol consumption and active or passive cigarette smoking have been commonly associated. Hence, increasing alcohol consumption irrespective of age leads to syndrome-like ARDS. Other risk factors like dyspnoea, hypertension, diabetes, and more lead to ARDS development. Thereby, this wide range of risk factors associated with acute respiratory distress syndrome increases the possibility of developing the clinical condition, simultaneously raising the cases of the disease. These affirmative risk factors are anticipated to boost the growth of the market.
- Acceleration in patient pool of covid-19 with Acute Respiratory Distress Syndrome (ARDS)
As the world is suffering enormously from COVID-19 infection, there is also a direct correlation between COVID-19 and acute respiratory distress syndrome. Severe cases of COVID-19 disease will ultimately lead to ARDS and pneumonia. This has been proven to be fatal for infected individuals. When the COVID-19-causing virus enters the body, it attaches to the upper airway cells. This sets off an immune response that causes inflammation and leads to symptoms such as cough, sore throat, and fever. In some severe cases, the virus travels beyond the upper airway, moves through the lungs, and ends up in the alveoli. Thereby, the rising number of COVID-19 cases also increases the chance of acute respiratory distress syndrome among people, which is expected to act as a driver for the growth of the acute respiratory distress syndrome market.
- The rising rate of air pollution and lifestyle-related diseases
Long-term exposure to low to moderate air pollution is associated with a greater risk factor for developing any lung infection, including ARDS. This is a novel and potentially modifiable environmental risk factor for acute respiratory distress syndrome. According to the World Health Organization, air pollution is one of the greatest environmental risks to health by increasing the burden of diseases like heart diseases and lung and respiratory infections at both acute and chronic levels. Hence, the rising air pollution rate in developed and developing countries increases the chances of people getting syndromes like acute respiratory distress syndrome. Thus, the factor is expected to drive the growth of the market.
- Increasing accident rates and trauma causing Acute Respiratory Distress Syndrome (ARDS)
Major trauma caused by accidents is a well-known risk factor for the development of ARDS. Acute respiratory distress syndrome can result from an injury to the body by creating inflammation of the pancreas, breathing contents of the stomach into the lung, blood transfusions, house fire and smoke inhalation, serious infection, drowning trauma, and severe reaction to medications. Even car accidents are a cause of ARDS. Hence, inhalation of smoke releases certain materials like fibrin, neutrophils, mucus, and epithelial cell debris that occlude the airway lumen, causing changes in ventilation. This condition leads to hypoxemia which is a primary significant reason for ARDS.
Opportunities
- Growing geriatric population
The 'world's geriatric population is increasing at a rapid rate. Older people have become an increasingly prevalent population of intensive care admissions. As per an article on National Center for Biotechnology Information (NCBI), the mortality rate associated with acute respiratory distress syndrome (ARDS) has been 69% to 80% reported among the elderly population, making it riskier for geriatric people. Thus, the growing pace of the older population demonstrates the escalation in demand for acute respiratory distress syndrome treatments as they are more vulnerable to severe respiratory illness due to their weak immune system. It is anticipated that the -increasing geriatric population, which is expected to rise in future years worldwide, creates an opportunity for market growth in the forecast period.
- Rising healthcare expenditure
Healthcare expenditure has increased worldwide as people's disposable income in various countries is increasing. Moreover, to accomplish the population requirements, the government bodies and healthcare organizations are taking the initiative by virtue of accelerating healthcare expenditure. The rise in healthcare expenditure simultaneously helps healthcare settings to improve their treatment facilities for acute respiratory distress syndrome, as the disorder has been highly prevalent in recent years. Growing healthcare expenditure is also beneficial for further economic and healthcare sector growth. It is primarily fruitful as it significantly affects the development of better and advanced therapeutic options with ventilators and other ARDS treatment devices.
- Strategic initiatives by market players
With increasing rates of infectious diseases such as COVID-19 and chronic diseases like lung cancer, the simultaneous burden of 'syndrome like ARDS is widely seen globally. The dramatic rise in research quality and increasing research opportunities is because of various strategic initiatives these players take. They are taking initiatives such as product launches, collaborations, mergers, acquisitions, and many more over the years and are expected to lead and create more opportunities in the market. These strategic product launches, acquisitions, and mergers done by major companies in the acute respiratory distress syndrome market have opened up opportunities for companies in various regions. This strategy is allowing the companies to strengthen their footprints in the market.
- Improving awareness regarding Acute Respiratory Distress Syndrome (ARDS)
Since acute respiratory distress syndrome has multiple different causes, it is usually ignored under common causes of death. The requirement of advanced technical treatment and proper awareness of the condition can substantially decline ARDS incidence. As timely diagnosis and prevention are crucial to preventing or recovering faster, the public's attention is most important. Current government and organizations have broadened the scope of lung injury research to include primary prevention of ARDS and reduce the morbidity or mortality rate for the syndrome. Thereby, increasing awareness regarding ARDS by diverse 'association's support enhances the U.S. acute respiratory distress syndrome 'market's opportunity for wide growth in the future.
Restraints/Challenges
- Complications associated with treatments
Acute respiratory distress syndrome is a clinical condition requiring proper and timely treatment and cares to prevent mortality and morbidity rates. However, during the treatment of ARDS, the patients might face many complications in the hospitals. Though there are treatments for ARDS helping people survive, the complications associated during treatments and post-treatment are still a burden for patients. Most potential and lasting effects include breathing problems, depression, problems with memory and thinking, tiredness and muscle weakness, and more. These complications associated with and post-treatment of acute respiratory distress syndrome are expected to hamper the market growth during our forecast period.
- High cost of device and treatments
Though acute respiratory distress syndrome is getting a wide range of advanced treatment options, the cost of treatment for longer is quite difficult for average-income people to afford. The utilization of critical care and intensive care unit services is increasing worldwide, and its expensive cost is a major concern in the current healthcare system. Patients with ARDS are commonly required to have long hospitalizations with frequent monetarization and ventilation usage, consuming a significant amount of healthcare resources. Therefore, the high cost of treatment and ventilators is very challenging for people to afford any reliable remedy for the long term until the patient completely recovers. This is anticipated to hamper the growth of the market.
- Lack of skilled workforce
The sudden COVID-19 crisis has created various challenges to be faced by a healthcare organization, including a workforce shortage. The pandemic massively struck developed countries like the U.S. They initiated multiple ways to overcome such challenges, but news articles reported that the countries were dealing with staffing shortages. There were shortages of nurses even before the pandemic began in the U.S., as the region has a high population with respiratory disorders. Therefore, the lack of appropriately skilled and trained nurses in the ICU will be a more remarkable restraint expected to hamper the growth of the acute respiratory distress syndrome (ARDS) market.
- Stringent rules & regulations
The use of ventilators across the globe is rapidly increasing, with the growth of the aged population and several acute lung diseases, including acute respiratory distress syndrome, which is preventable by early diagnosis and timely treatments. At the same time, the players of the acute respiratory distress syndrome manufacturers in the market have to follow specific regulations to get approval from the upper authorities to launch the product like mechanical ventilators or humidifiers. These stringent guidelines need to be followed, and this is one of the most difficult tasks among all the steps. For example, the U.S. Food and Drug Administration (FDA) regulates respiratory ailment products in the U.S. Therefore, the stringent rules & regulations for product approval challenge the market's growth.
- Multiple challenges faced by ICU nurses
The intensive care units have many challenges to face during the pandemic due to a large patient pool and fewer hospital resources. Since the first wave of COVID-19, the hospitals have increased the capacity and potential of 'ICUs, which has led to increased working hours for ICU health care providers. The nurses dealing in ICU are always in fear due to multiple reasons for handling infected patients. Therefore, the most significant challenge is the difficulties faced by nurses and other healthcare providers dealing with acute respiratory distress syndrome and severe acute respiratory syndrome. Organizations are trying to overcome them with new innovative ideas and appointing more workforces.
Recent Development
- In July 2020, Gilead Sciences, Inc. initiated clinical testing of an Inhaled Solution of Remdesivir for Potential Outpatient Treatment of COVID-19. This testing helped the company enhance its antiviral product portfolio, thereby strengthening its footprint on the market
U.S. Acute Respiratory Distress Syndrome (ARDS) Market Scope
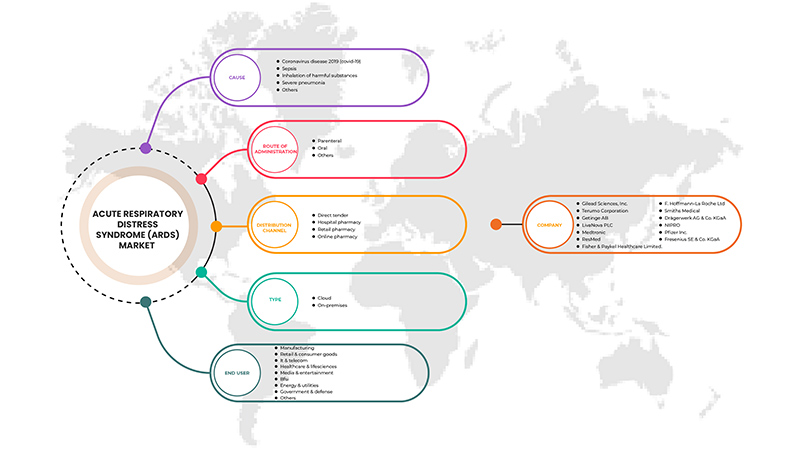
U.S. acute respiratory distress syndrome (ARDS) market is categorized based on cause, type, route of administration, end-user and distribution channel. The growth amongst these segments will help you analyze major growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Cause
- Coronavirus Disease 2019 (COVID-19)
- Sepsis
- Inhalation Of Harmful Substances
- Severe Pneumonia
- Others
Based on the cause, the U.S. acute respiratory distress syndrome (ARDS) market is classified into coronavirus disease 2019 (COVID-19), sepsis, inhalation of harmful substances, severe pneumonia, and others.
Type
- Diagnosis
- Treatment
Based on type, the U.S. acute respiratory distress syndrome (ARDS) market is classified into diagnosis and treatment.
Route of Administration
- Oral
- Parenteral
- Others
Based on route of administration, the U.S. acute respiratory distress syndrome (ARDS) market is classified into oral, parenteral, and others.
End User
- Hospitals
- Specialty Clinics
- Home Healthcare
- Others
Based on end-user, the U.S. acute respiratory distress syndrome (ARDS) market is classified into hospitals, specialty clinics, home healthcare, and others.
Distribution Channel
- Direct Tender
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Based on distribution channel, the U.S. acute respiratory distress syndrome (ARDS) market is classified into direct tender, hospital pharmacy, retail pharmacy, online pharmacy, and others.
Competitive Landscape and U.S. Acute Respiratory Distress Syndrome (ARDS) Market Share Analysis
U.S. acute respiratory distress syndrome (ARDS) market competitive landscape provides details of competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the companies' focus related to the U.S. acute respiratory distress syndrome (ARDS) market.
Some of the prominent participants operating in the U.S. acute respiratory distress syndrome (ARDS) market are Gilead Sciences, Inc., Terumo Medical Corporation, Getinge AB., Medtronic, LivaNova PLC, Fresenius SE & Co. KGaA, Drägerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd, Fisher & Paykel Healthcare Limited., Hamilton Medical, NIPRO, Pfizer Inc., ResMed, Smiths Medical, WEINMANN Emergency Medical Technology GmbH + Co. KG.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by the DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning grids, Market Time Line Analysis, Market Overview and Guide, Company Positioning grids, Company Market Share Analysis, Standards of Measurement, U.S. Vs. Regional, and Vendor Share Analysis. Please request an analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CAUSE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
4.3 PIPELINE ANALYSIS
5 INSURANCE REIMBURSEMENT
5.1 CENTER FOR MEDICARE SERVICES (CMS)–ELSO (EXTRACORPOREAL LIFE SUPPORT ORGANIZATION)
5.2 HEALTH RESOURCES AND SERVICES ADMINISTRATION
5.3 ABBOTT CODING GUIDE FOR ECMO
5.4 CERN HEALTH INSURANCE SCHEME
5.5 AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) – (MEDICARE & MEDICAID)
5.6 AMERICAN HOSPITAL ASSOCIATION
5.7 CONCLUSION
6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: REGULATIONS
6.1 REGULATION IN U.S.
6.2 REGULATION FOR VENTILATORS AND RESPIRATORY DEVICES AS PER FDA
6.3 LABELING OF MODIFIED DEVICES
7 COUNTRY SUMMERY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASING PREVALENCE AND INCIDENCE OF ACUTE LUNG INJURY
8.1.2 WIDE RANGE OF RISK FACTORS FOR ARDS
8.1.3 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS
8.1.4 RISING RATE OF AIR POLLUTION AND LIFESTYLE-RELATED DISEASES
8.1.5 INCREASING ACCIDENT RATES AND TRAUMA CAUSING ARDS
8.2 RESTRAINTS
8.2.1 COMPLICATIONS ASSOCIATED WITH TREATMENTS
8.2.2 HIGH COST OF DEVICE AND TREATMENTS
8.2.3 LACK OF SKILLED WORKFORCE
8.3 OPPORTUNITIES
8.3.1 GROWING GERIATRIC POPULATION
8.3.2 RISING HEALTHCARE EXPENDITURE
8.3.3 STRATEGIC INITIATIVES BY MARKET PLAYERS
8.3.4 IMPROVING AWARENESS REGARDING ARD SYNDROME
8.4 CHALLENGES
8.4.1 STRINGENT RULES & REGULATIONS
8.4.2 MULTIPLE CHALLENGES FACED BY ICU NURSES
9 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE
9.1 OVERVIEW
9.2 CORONAVIRUS DISEASE 2019 (COVID-19)
9.3 SEPSIS
9.4 INHALATION OF HARMFUL SUBSTANCES
9.5 SEVERE PNEUMONIA
9.6 OTHERS
10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE
10.1 OVERVIEW
10.2 DIAGNOSIS
10.2.1 IMAGING TESTS
10.2.1.1 CHEST X-RAY
10.2.1.2 CT SCAN
10.2.1.3 ULTRASOUND
10.2.1.4 OTHERS
10.2.2 BLOOD TEST
10.2.3 RESPIRATORY RATE
10.2.4 SPO2 TEST
10.2.5 OTHERS
10.3 TREATMENT
10.3.1 MECHANICAL VENTILATION
10.3.1.1 HIGH-FLOW NASAL O2
10.3.1.2 BI-LEVEL POSITIVE AIRWAY PRESSURE
10.3.1.3 CONTINOUS POSITIVE AIRWAY PRESSURE
10.3.1.4 PRONE POSITIVE VENTILATION
10.3.1.5 OTHERS
10.3.2 CORTICOSTEROIDS
10.3.2.1 METHYLPREDNISOLONE
10.3.2.2 DEXAMETHASONE
10.3.2.3 OTHERS
10.3.3 ANTIVIRAL MEDICATION
10.3.3.1 REMDESIVIR
10.3.3.2 COMBINATION DRUGS
10.3.3.3 OTHERS
10.3.4 EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
10.3.5 TOCILIZUMAB
10.3.6 OTHERS
11 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION
11.1 OVERVIEW
11.2 PARENTERAL
11.2.1 INTRAVENOUS
11.2.2 INTRAMUSCULAR
11.3 ORAL
11.4 OTHERS
12 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 SPECIALTY CLINICS
12.4 HOME HEALTHCARE
12.5 OTHERS
13 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 HOSPITAL PHARMACY
13.4 RETAIL PHARMACY
13.5 ONLINE PHARMACY
14 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: U.S
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 GILEAD SCIENCES INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUS ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENT
16.2 TERUMO CORPORATION
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUS ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENT
16.3 GETINGE AB
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUS ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 LIVANOVA PLC
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 MEDTRONIC
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 DRÄGERWERK AG & CO. KGAA
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 F. HOFFMANN-LA ROCHE LTD
16.7.1 COMPANY SNAPSHOT
16.7.2 RECENT ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 FISHER & PAYKEL HEALTHCARE LIMITED
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 FRESENIUS SE & CO. KGAA
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUS ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 HAMILTON MEDICAL
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 NIPRO
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUS ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 PFIZER INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUS ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 RESMED
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 SMITHS MEDICAL
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUS ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH + CO. KG
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: PIPELINE ANALYSIS
TABLE 2 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 3 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 4 U.S. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 5 U.S. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 6 U.S. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 U.S. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 8 U.S. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 U.S. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 11 U.S. PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 12 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 13 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 2 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DATA TRIANGULATION
FIGURE 3 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DROC ANALYSIS
FIGURE 4 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COUNTRY MARKET ANALYSIS
FIGURE 5 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 11 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS IS EXPECTED TO DRIVE THE U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
FIGURE 14 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2021
FIGURE 15 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2022-2029 (USD MILLION)
FIGURE 16 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2022-2029)
FIGURE 17 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 18 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2021
FIGURE 19 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 20 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 21 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 23 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD MILLION)
FIGURE 24 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 25 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 26 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2021
FIGURE 27 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 28 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2022-2029)
FIGURE 29 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DISTRIBUTION CHANNEL, 2021
FIGURE 31 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 32 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 33 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












