North America Thyroid Cancer Diagnostics Market Analysis and Insights
Increasing awareness about thyroid cancer has enhanced the market demand. The rising healthcare expenditure for better health services also contributes to the market's growth. The major market players focus on various service launches and approvals during this crucial period. In addition, the increase in improved diagnostic processes and techniques also contributes to the rising demand for thyroid cancer diagnostics testing.
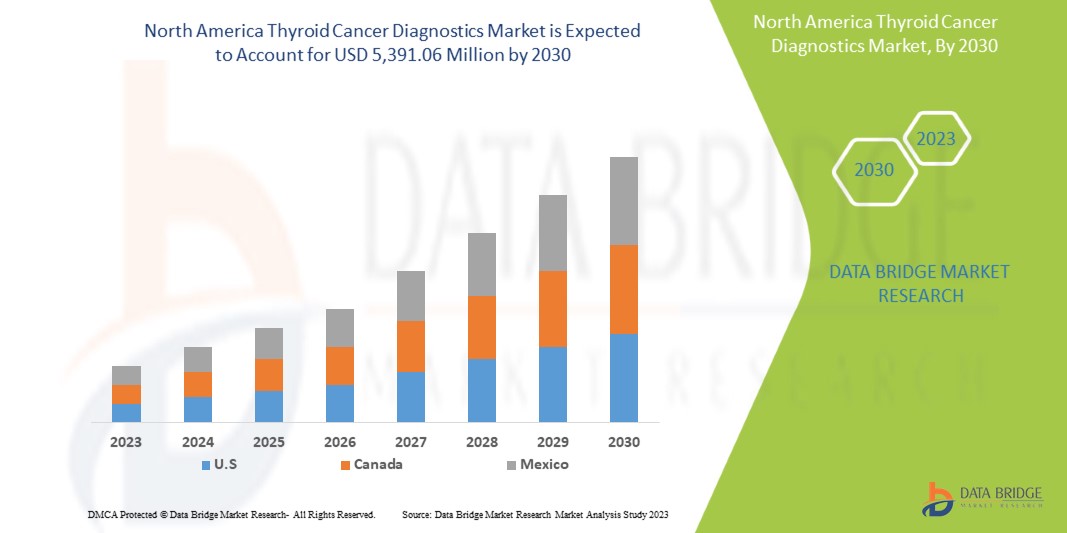
The North America thyroid cancer diagnostics market is expected to grow in the forecast period of 2023 to 2030. Data Bridge Market Research analyses that the market is growing with a CAGR of 6.4% in the forecast period of 2023 to 2030 and is expected to reach USD 5,391.06 million by 2030.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customisable to 2020-2015) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Product Type (Instruments, Consumables & Accessories), Test Type (Imaging Test, Biopsy, Blood Test, Others), Cancer Type (Papillary Carcinoma, Follicular Carcinoma, Others), Stages (Stage I, Stage II, Stage III, Stage IV), Age Group (Below 21, 21-29, 30-65, 65 and Above), End User (Hospital, Associated Labs, Independent Diagnostic Laboratories, Diagnostic Imaging Centers, Cancer Research Institutes, and Others), Distribution Channel (Direct Tender, Retail Sales) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
Canon Inc., FUJIFILM Holdings Corporation, F. Hoffmann-La Roche Ltd, Quest Diagnostics Incorporated, Illumina, Koninklijke Philips N.V., Thermo Fisher Scientific Inc., Siemens Healthcare GmbH, Abbott, General Electric Company, BD, QIAGEN, DIASORIN S.P.A., Merck KGaA, Hologic, Myriad Genetics Inc., BIOMERIEUX, FONAR Corp., Time Medical Holding., PlexBio., MinFound Medical Systems Co., Ltd, Medonica Co. LTD, Beijing O&D Biotech Co., Ltd., and SternMed GmbH. among others |
Market Definition
Thyroid cancer is a type of cancer that starts in the thyroid gland. Cancer starts when cells begin to grow out of control. The thyroid gland makes hormones that help regulate your metabolism, heart rate, blood pressure, and body temperature. The thyroid gland is in the front part of the neck, below the thyroid cartilage (Adam's apple). In most people, the thyroid cannot be seen or felt. It is shaped such as a butterfly, with two lobes — the right lobe and the left lobe — joined by a narrow piece of the gland called the isthmus.
North America Thyroid Cancer Diagnostics Market Dynamics
DRIVERS
RISING INCIDENCE AND PREVALENCE OF THYROID NODULES & CANCER
A thyroid nodule is an unusual growth (lump) of thyroid cells in the thyroid gland. Sometimes the normal thyroid soft tissue started to grow, causing these nodules to form. Nodule incidence increases with age and mainly in women, mainly in those with iodine deficiency and after radiation exposure. Although the further complication of these nodules is thyroid cancer, the chances of converting thyroid nodules to thyroid cancer are low. According to an article published in NCBI named "Risk of Malignancy in Thyroid Nodules 4 cm or Larger" in 2017, cancer occurrence after nodules is found in less than 5% of the total nodules cases. Moreover, the thyroid nodules run in the family history and in people whose iodine intake is low.
RISING THYROID CANCER DIAGNOSTIC TESTS
Thyroid ultrasound is a sound wave picture of the thyroid gland taken by a hand-held instrument and translated to a 2-dimensional picture on a monitor. It is used in the diagnosis of tumors, cysts, or goiters of the thyroid and is a painless, no-risk procedure. These tests are used to evaluate structural anomalies, whereas blood tests measuring TSH, T4, and T3 levels are used to examine functional variables. Fine needle biopsy is used in suspicious cases to determine whether a tumor is benign or malignant. Furthermore, the introduction of molecular testing and the genetic prognosis fuel the diagnostic landscape in this market for thyroid cancer diagnostics.
RISING AWARENESS TOWARDS THYROID CANCER
Growing thyroid cancer awareness has led to an increased demand for timely cancer detection, leading to market growth.
Thyroid cancer is one of the major causes of rising mortality rates among U.S. populations worldwide, fueling the market growth over the next five years. Exposure to radiation and a family history of thyroid issues are major risk factors for thyroid cancer. Women are diagnosed with thyroid cancer significantly more than men.
RESTRAINS
HIGH COST OF DIAGNOSTICS PROCEDURE
Cancer diagnostics have become increasingly expensive due to the growing number of thyroid cancer patients and the rising medical device prices. The modern technological devices used in cancer diagnostic is also playing a significant role in the high prices of cancer diagnostics, and high accuracy, in providing a definitive diagnosis for cancer in thyroid nodules. Therefore, the high cost of diagnostics procedures for thyroid cancers is hampering the growth of the market.
The diagnostic products or devices which are used in the detection of cancer are becoming advanced but along with that, the procedure for cancers diagnostics are also costly, which hamper the growth of the thyroid cancer diagnostics market because the devices are used in the process of cancer diagnosis getting more expensive which results to increase in the cost of diagnostics procedure. Thus, the high cost of diagnostics for cancer diagnosis acts as a restraint for the North America thyroid cancer diagnostics market.
TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
High radiation exposure causes significant tissue damage and raise a person's chance of later acquiring cancer. Although it's vital to keep this risk in context, the tiny radiation doses used for imaging tests may marginally raise a person's risk of developing cancer. The amount of radiation a person receives varies on the test's kind, the area of their body that is exposed, their body size, age, and gender, among other things. Imaging examinations that employ radiation should only be performed when necessary because radiation exposure from all sources can mount up over a lifetime and can increase the chance of developing cancer. Other imaging procedures like ultrasound or MRI may also be utilized often.
OPPORTUNITIES
RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
Across the globe, R&D activities are escalating owing to public health expenditure with economic performances whereas, the healthcare industry ranks second among all industries when it comes to the amount spent on healthcare. Rising healthcare expenditure can result in better provision of R&D opportunities. It is anticipated to upsurge the demand for ovarian cancer diagnostics.
Increasing healthcare expenditure for cancer treatment also helps patients to take hassle-free advanced diagnostics and treatment for fast recovery. Healthcare spending is made up of out-of-pocket payments (people paying for their own care), government expenditure, and sources, including health insurance and activities by Non-Governmental Organizations (NGOs). Due to this increasing healthcare expenditure for cancer treatment, it acts as an opportunity for market growth.
CHALLENGES
STRINGENT REGULATORY FRAMEWORK FOR THE APPROVAL AND COMMERCIALIZATION OF CANCER DIAGNOSTIC PRODUCTS
The stringent regulations for the approval and commercialization of any product in the market are proving to be one of the major challenges for manufacturers of cancer diagnostic products in the U.S. and European region. Every country has regulations and a different body for regulatory procedures.
The potential regulatory pathways are mandatory for clearance, approval, or acceptance of complex signatures by the U.S. Food and Drug Administration (FDA). The regulatory pathways include regulations applicable to In Vitro Diagnostic (IVD) devices, including companion diagnostic devices, the potential for labeling as a complementary diagnostic, and the biomarker qualification program.
Recent Developments
- In August 2022, F. Hoffmann-La Roche Ltd, announced the launch of the Digital LightCycler System, Roche's first digital polymerase chain reaction (PCR) system. This next-generation system detects disease and is designed to accurately quantify trace amounts of specific DNA and RNA targets not typically detectable by conventional PCR methods. This has helped the company to increase its North America presence in the market
- In May 2022, Thermo Fisher Scientific Inc., the world leader in serving science, introduced the Thermo Scientific Glacios 2 Cryo-Transmission Electron Microscope (Cryo-TEM), a powerful microscope with new automation and high-resolution imaging capabilities designed to help cryo-electron microscopy (cryo-EM) researchers of varying experience levels accelerate structure-based drug discovery. This advanced, fast, and cost-efficient method for drug design may enable customers to accelerate the pace of research for debilitating disorders like Alzheimer's, Parkinson's, and Huntington's diseases, as well as research for cancer and gene mutations
North America Thyroid Cancer Diagnostics Market Scope
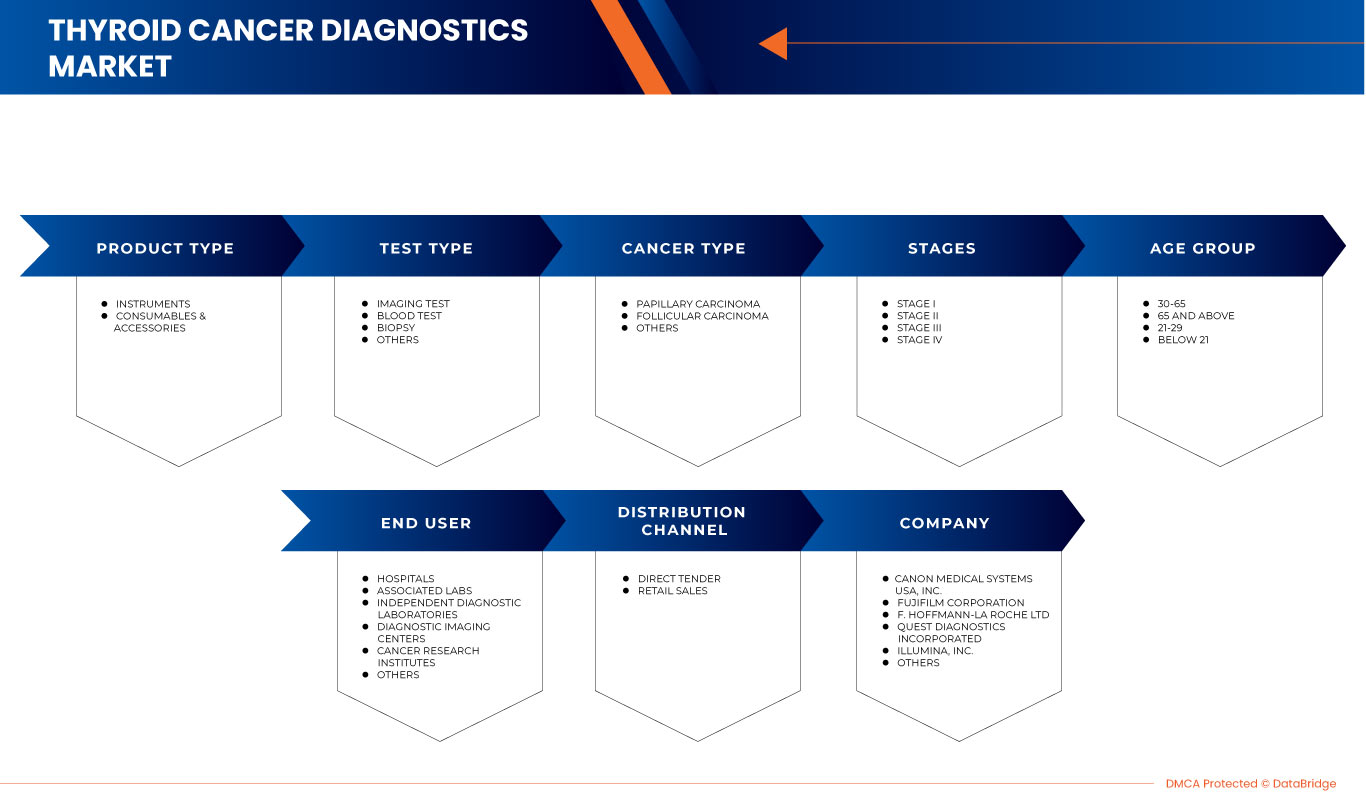
The North America thyroid cancer diagnostics market is segmented into product type, test type, cancer type, stages, age group, end user, and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Product Type
- Instruments
- Consumables & Accessories
On the basis of product type, the North America thyroid cancer diagnostics market is segmented into instruments and consumables & accessories.
Test Type
- Imaging Test
- Blood Test
- Biopsy
- Others
On the basis of test type, the North America thyroid cancer diagnostics market is segmented into imaging test, blood test, biopsy and others.
Cancer Type
- Papillary Carcinoma
- Follicular Carcinoma
- Others
On the basis of cancer type, the North America thyroid cancer diagnostics market is segmented into papillary carcinoma, follicular carcinoma, and others.
Stages
- Stage I
- Stage II
- Stage III
- Stage IV
On the basis of stages, the North America thyroid cancer diagnostics market is segmented into stage I, stage II, stage III and stage IV.
Age Group
- 30-65
- 65 and above
- 21-29
- Below 21
On the basis of age group, the North America thyroid cancer diagnostics market is segmented into 30-65, 65 and above, 21-29, and below 21.
End User
- Hospitals
- Associated Labs
- Independent Diagnostic Laboratories
- Diagnostic Imaging Centers
- Cancer Research Institutes
- Others
On the basis of end user, the North America thyroid cancer diagnostics market is segmented into hospitals, associated labs, independent diagnostic laboratories, diagnostic imaging centers, cancer research institutes, and others.
Distribution Channel
- Direct Tender
- Retail Sales
On the basis of distribution channel, the North America thyroid cancer diagnostics market is segmented into direct tender and retail sales.
North America Thyroid Cancer Diagnostics Market Regional Analysis/Insights
The North America thyroid cancer diagnostics market is analysed, and market size insights and trends are provided by country, product type, test type, cancer type, stages, age group, end user, and distribution channel, as referenced above.
The countries covered in this market report are U.S., Canada, and Mexico.
The U.S. is expected to dominate the North America thyroid cancer diagnostics market in terms of market share and revenue and will continue to flourish its dominance during the forecast period. This is due to rising thyroid cancer diagnostic tests.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of North American brands and the challenges faced due to competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Thyroid Cancer Diagnostics Market Share Analysis
North America thyroid cancer diagnostics market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the North America thyroid cancer diagnostics market.
Some of the major players operating in the North America thyroid cancer diagnostics market are Canon Inc., FUJIFILM Holdings Corporation, F. Hoffmann-La Roche Ltd, Quest Diagnostics Incorporated, Illumina, Koninklijke Philips N.V., Thermo Fisher Scientific Inc., Siemens Healthcare GmbH, Abbott, General Electric Company, BD, QIAGEN, DIASORIN S.P.A., Merck KGaA, Hologic, Myriad Genetics Inc., BIOMERIEUX, FONAR Corp., Time Medical Holding., PlexBio., MinFound Medical Systems Co., Ltd, Medonica Co. LTD, Beijing O&D Biotech Co., Ltd., and SternMed GmbH. among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GROWTH STRATEGIES ADOPTED BY KEY MARKET PLAYERS
5 EPIDEMIOLOGY
6 REGULATORY FRAMEWORK OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
6.1 REGULATORY SCENARIO IN THE U.S.
6.2 REGULATORY SCENARIO IN AUSTRALIA
6.3 REGULATORY SCENARIO IN JAPAN
6.4 REGULATORY SCENARIO IN CHINA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISING INCIDENCE AND PREVALENCE OF THYROID NODULES AND CANCER
7.1.2 RISING THYROID CANCER DIAGNOSTIC TESTS
7.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.1.4 RISING AWARENESS TOWARDS THYROID CANCER
7.2 RESTRAINTS
7.2.1 HIGH COST OF DIAGNOSTICS PROCEDURE
7.2.2 TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
7.3 OPPORTUNITIES
7.3.1 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.3.2 RISING OBESE POPULATION
7.4 CHALLENGES
7.4.1 STRINGENT REGULATORY FRAMEWORK FOR THE APPROVAL AND COMMERCIALIZATION OF CANCER DIAGNOSTIC PRODUCTS
7.4.2 LACK OF SKILLED AND CERTIFIED EXPERTISE
8 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 INSTRUMENTS
8.2.1 PATHOLOGY BASED INSTRUMENTS
8.2.1.1 PCR INSTRUMENTS
8.2.1.2 SLIDE STAINING SYSTEMS
8.2.1.3 TISSUE PROCESSING SYSTEMS
8.2.1.4 CELL PROCESSORS
8.2.1.5 OTHER PATHOLOGY-BASED INSTRUMENTS
8.2.2 IMAGING INSTRUMENTS
8.2.2.1 ULTRASOUND SYSTEMS
8.2.2.2 CT SYSTEMS
8.2.2.3 MRI SYSTEMS
8.2.2.4 OTHERS
8.2.3 BIOPSY INSTRUMENTS
8.2.3.1 NEEDLE BIOPSY
8.2.3.2 ENDOSCOPIC BIOPSY
8.2.3.3 CORE BIOPSY
8.2.3.4 OTHERS
8.2.4 OTHERS
8.3 CONSUMABLES & ACCESSORIES
8.3.1 KITS
8.3.1.1 PCR KITS
8.3.1.2 DNA POLYMERASE KITS
8.3.1.3 NUCLEIC ACID ISOLATION KITS
8.3.1.4 OTHERS
8.3.2 REAGENTS
8.3.2.1 ASSAYS
8.3.2.2 BUFFERS
8.3.2.3 PRIMERS
8.3.2.4 OTHERS
8.3.3 PROBES
8.3.4 OTHER CONSUMABLES
9 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.2 MRI
9.2.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
9.2.4 OTHERS
9.3 BLOOD TEST
9.3.1 BLOOD CHEMISTRY TESTS
9.3.2 COMPLETE BLOOD COUNT (CBC)
9.3.3 OTHERS
9.4 BIOPSY
9.4.1 NEEDLE BIOPSY
9.4.2 BRONCHOSCOPY BIOPSY
9.4.3 CORE BIOPSY
9.4.4 OTHERS
9.5 OTHERS
10 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 PAPILLARY CARCINOMA
10.3 FOLLICULAR CARCINOMA
10.4 OTHERS
11 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES
11.1 OVERVIEW
11.2 STAGE I
11.3 STAGE II
11.4 STAGE III
11.5 STAGE IV
12 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP
12.1 OVERVIEW
12.2 30-65
12.3 65 AND ABOVE
12.4 21-29
12.5 BELOW 21
13 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.3 ASSOCIATED LABS
13.4 INDEPENDENT DIAGNOSTIC LABORATORIES
13.5 DIAGNOSTIC IMAGING CENTERS
13.6 CANCER RESEARCH INSTITUTES
13.7 OTHERS
14 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL SALES
15 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 CANON INC.
18.1.1 COMPANY SNAPSHOT
18.1.2 COMPANY SHARE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 FUJIFILM CORPORATION
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENT
18.3 F. HOFFMANN-LA ROCHE LTD
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 QUEST DIAGNOSTICS INCORPORATED
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 ILLUMINA, INC.
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBOTT
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 BD
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 BEIJING O&D BIOTECH CO., LTD.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 BIOMÉRIEUX SA
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENTS
18.1 DIASORIN S.P.A.
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 FONAR CORP.
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENTS
18.12 GENERAL ELECTRIC
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 HOLOGIC INC.
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 KONINKLIJKE PHILIPS N.V.
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 MERCK KGAA.
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MEDONICA CO. LTD
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MINFOUND MEDICAL SYSTEMS CO. LTD
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENTS
18.18 MYRIAD GENETICS, INC.
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENTS
18.19 PLEXBIO.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENTS
18.2 QIAGEN
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 STERNMED GMBH
18.21.1 COMPANY SNAPSHOT
18.21.2 PRODUCT PORTFOLIO
18.21.3 RECENT DEVELOPMENTS
18.22 SIEMENS HEALTHCARE GMBH
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENT
18.23 TIME MEDICAL HOLDING.
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 THERMO FISHER SCIENTIFIC INC.
18.24.1 COMPANY SNAPSHOT
18.24.2 REVENUE ANALYSIS
18.24.3 PRODUCT PORTFOLIO
18.24.4 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 2 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA PAPILLARY CARCINOMA IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA FOLLICULAR CARCINOMA IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA STAGE I IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA STAGE II IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA STAGE III IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA STAGE IV IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA 30-65 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA 65 AND ABOVE IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA 21-29 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA BELOW 21 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA HOSPITALS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA ASSOCIATED LABS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA INDEPENDENT DIAGNOSTIC LABORATORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA DIAGNOSTIC IMAGING CENTERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA CANCER RESEARCH INSTITUTES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA DIRECT TENDER IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA RETAIL SALES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 61 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 U.S. INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 U.S. PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 U.S. IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 65 U.S. BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 66 U.S. CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 67 U.S. KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 68 U.S. REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 69 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 U.S. IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 U.S. BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 U.S. BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 74 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 75 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 76 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 78 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 CANADA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 CANADA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 CANADA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 CANADA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 CANADA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 CANADA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 85 CANADA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 86 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 CANADA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 88 CANADA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 89 CANADA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 90 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 91 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 92 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 93 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 94 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 95 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 MEXICO INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 MEXICO PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 MEXICO IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 MEXICO BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 MEXICO CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 MEXICO KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 MEXICO REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 103 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 MEXICO IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 MEXICO BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 MEXICO BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 108 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 109 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 110 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 111 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN THE AWARENESS ABOUT THYROID CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET IN THE FORECAST PERIOD
FIGURE 12 PRODUCT TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 19 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 23 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, 2022
FIGURE 27 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, LIFELINE CURVE
FIGURE 30 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, 2022
FIGURE 31 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 34 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 35 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 39 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 42 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 43 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 44 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 45 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 46 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: PRODUCT TYPE (2023-2030)
FIGURE 47 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.