North America Pancreatic Cancer Diagnostics Market Analysis and Insights
The growing prevalence of pancreatic cancer as well as increasing need for diagnostic products for these conditions have enhanced the market demand. The advancement in technology for easy supply of products and fast manufacturing facilities are also attributing in the growth of the market. The major market players are highly focusing on product launches and product approvals during this crucial period. In addition, the government and regulatory bodies are supporting market players by product approval due to surging emergence.
The North America pancreatic cancer diagnostics market is expected to grow in the forecast year due to the rise in market players and the availability of advanced services. Along with this, manufacturers are engaged in R&D activity for launching novel services in the market. The increasing research in the field of leukemia diagnosis and development is expected to further boost the market growth. However, difficulties in leukemia screening techniques is expected to hamper the growth of the North America pancreatic cancer diagnostics market in the forecast period. Increasing healthcare expenditure on cancer diagnosis and treatment is expected to give opportunities to the market to enhance the treatment. The improvement in awareness about regular healthcare checkups, upcoming diagnostic centers and advancements in diagnostic methods for pancreatic cancer and technological developments is expected to boost the market’s growth. However, the high cost of testing and strict regulations and standards for the approval and commercialization of cancer diagnostic products and instruments is expected to challenge market growth.
The growing geriatric population, strategic initiatives by market players and government, and surge in healthcare expenditure give the market opportunities to enhance the treatment. However, the lack of skilled professionals and stringent regulatory frameworks are key challenges for the market growth. However, high cost of devices and treatments is expected to restrain the growth of the North America pancreatic cancer diagnostics market.
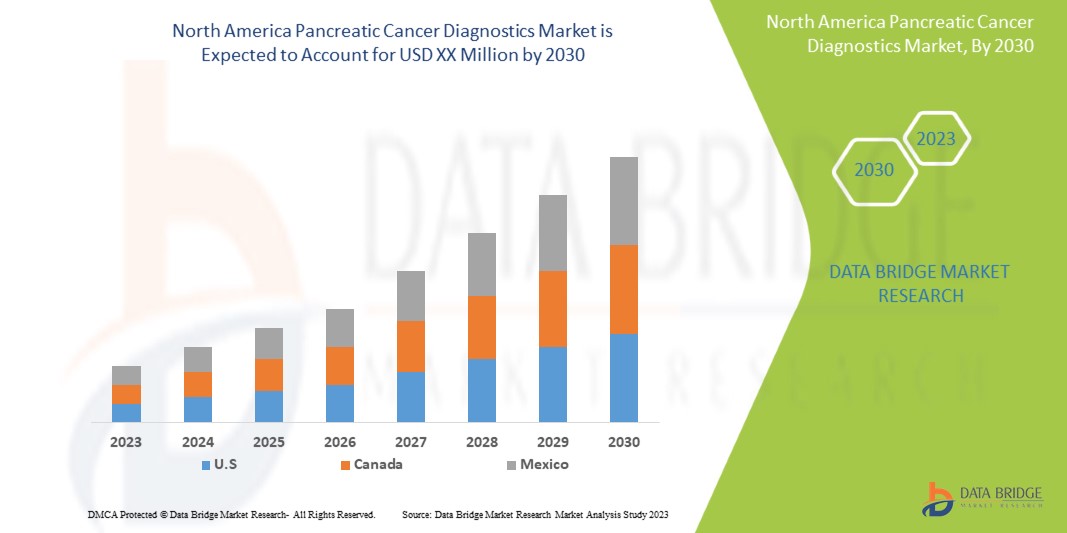
The North America pancreatic cancer diagnostics market is supportive and aims to reduce the disease thereby improving the recovery and performance of individuals. Data Bridge Market Research analyzes that North America pancreatic cancer diagnostics market will grow at a CAGR of 7.7% during the forecast period of 2023 to 2030.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2020-2016) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
By Test Type (Imaging Test, Biopsy, Blood Test, Genomic Test, and Others), Cancer Stage (Stage 0, Stage I, Stage II, Stage III and Stage IV), Tumor Type (Exocrine Tumors and Neuroendocrine Tumors), Product (Instrument-Based Products, Platform-Based Products, Kits and Reagents, and Other Consumables), Technology (Fluorescent In Situ Hybridization, Next Generation Sequencing, Fluoroimmunoassay, Comparative Genomic Hybridization, Immunohistochemical, and Others), Application (Screening, Diagnostic and Predictive, Prognostic, and Research), End User (Hospitals, Diagnostic Centers, Cancer Research Centers, Academic Institutes, Ambulatory Surgical Centers, and Others), Distribution Channel (Direct Tender, Retail Sales and Others). |
|
Country Covered |
U.S., Canada, Mexico. |
|
Market Players Covered |
Siemens Healthcare Private Limited, Koninklijke Philips N.V., FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, DiaSource, Abbott, Agilent Technologies, Inc., Lee Biosolutions, Inc, MP BIOMEDICALS, Setia Scientific Solution, Boditech Med Inc., AccuBioTech Co., Ltd., Thermo Fisher Scientific, Creative Biolabs, Myriad Genetics, Inc., BD, CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Meridian Life Science, Inc., CTK Biotech, Inc., among others |
Market Definition
Pancreatic cancer is fatal, and the diagnosis process of pancreatic cancer also has safety issues; it is not cost-effective. One of the most costly medical disorders to treat North America is cancer. Cancer patients may be hospitalized and receive a variety of therapies for tumor, such as surgery, radiation treatment, and systemic therapy. Health insurance premiums for cancer patients are now more expensive than in the past. In addition, their copayment, deductible, and coinsurance costs are rising. Diagnosis of pancreatic cancer includes ultrasound, biopsy procedures and blood tests. Pancreatic cancer is one of the leading causes of death worldwide, and the prevalence of this disease has increased at an alarming rate.
North America Pancreatic Cancer Diagnostics Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of these are discussed in detail below:
Drivers
- Grow in prevalence of pancreatic cancer
All ages can be affected by this type of cancer. Pancreatic cancer can be difficult to diagnose because, despite its wide range of signs and symptoms, they are non-specific and can be linked to other, more widespread medical conditions. Pancreatic cancer is the eighth most common cancer in women and the tenth most common cancer in men. Incidence rates of pancreatic cancer have gone up by around 1% each year. It occurs less frequently. It is slightly more common among women than men, however the average lifetime risk of getting pancreatic cancer in both sexes is about ½ of 1% on average. These conditions include: abdominal pain, loss of appetite or unintended weight loss, yellowing of skin and the whites of eyes (jaundice), light-colored stools, dark-colored urine and itchy skin.It is the 8th most common type of cancer diagnosed in adults and children, but most cases occur in adults. Although it can be diagnosed at any age, it is uncommon before age 45. The average age of diagnosis is age 68.
Due to various risk factors, pancreatic cancer incidence has been rising North America, becoming a significant socio-economic issue. This is expected to act as a driver in the North America pancreatic cancer diagnostics market.
- Novel technological advancements in pancreatic diagnostics
Pancreatic cancer is seldom detected at its early stages when it's most curable. This is because it often doesn't cause symptoms until after it has spread to other organs. Specialists must manually diagnose cancer and non-cancer cells by examining cell images under a microscope and providing labels through annotation. However, this hand microscopic examination is time-consuming and could give an incorrect diagnosis. The risk of prescribing the incorrect drugs was then reduced using computerized software. The creation of an automatic and reliable classification system became vital to stop the pancreatic disease's devastating effects. Multiple segmentation techniques constituted the foundation of the existing pancreatic cancer classification algorithms.
Opportunity
- Rise in healthcare expenditure for cancer diagnosis and treatment
Across the globe, research and development activities are escalating owing to the public health expenditure with economic performances. Whereas the healthcare industry ranks second among all industries when it comes to the amount spent on healthcare. Rising healthcare expenditure can result in better provision of research and development opportunities. It is anticipated to increase the demand for pancreatic cancer diagnostics. Increasing the healthcare expenditure for pancreatic cancer treatment also helps the patient take hassle-free advanced diagnostics and treatment for fast recovery. The spending on healthcare is made up of the combination of out-of-pocket payments (people paying for their care), government expenditure, and sources. It also includes health insurance and activities by non-governmental organizations. This increasing healthcare expenditure for cancer treatment is an opportunity for the market's demand.
Restraint/Challenge
- Late diagnosis and poor prognosis of pancreatic cancer
Late diagnosis of disease is due to the increasing pancreatic cancer tumors which do not respond as well to commonly used cancer therapies as other, less lethal types of cancer. But there are treatment options, including surgery, chemotherapy and radiation. There are different types of pancreatic cancer. Most pancreatic cancers are the exocrine type. This means that they start in cells that produce pancreatic digestive juices. About 30 percent of patients are smokers, and 5 percent have a history of pancreatitis, an inflammation of the pancreas, which can be caused by stones or heavy alcohol intake.
Post COVID-19 Impact on North America Pancreatic Cancer Diagnostics Market
COVID-19 has negatively affected the growth of the market as patients suffering for pancreatic cancer postponed their surgery due to rapid surge in covid-19 cases across geographies. Additionally, people having pancreatic cancer were at risk of becoming severely ill. The fear of corona virus infection affected the growth of the pancreatic cancer diagnostic market amid pandemic.
Recent Developments
- In December 2022, FUJIFILM Holdings America Corporation announced the company has purchase agreement asset with Inspirata, Inc. to acquire digital pathology business to expand robust enterprise imaging offering. This results in enabling the integration of pathology images and data into a healthcare organization’s electronic health record system to streamline care delivery for oncology patients.
- In August 2020, Siemens Healthcare GmbH announced that it has entered in to an agreement with Varian Medical Systems, Inc. With this acquisition, Siemens Healthcare has helped in developing advanced solutions to treat against cancer and strengthen their position in healthcare industry.
North America Pancreatic Cancer Diagnostics Market Scope
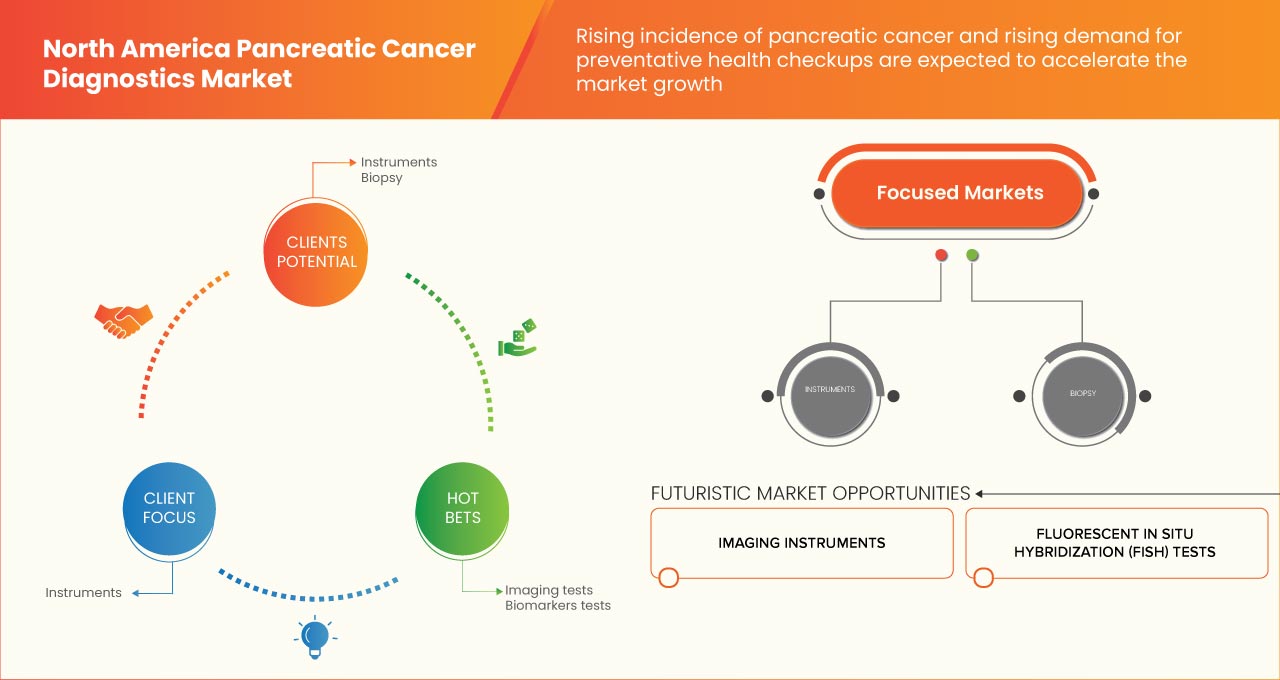
North America pancreatic cancer diagnostics market is categorized into eight notable segments based on test type, cancer stages, tumor type, product, application, technology, end user and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Test Type
- Imaging Test
- Biopsy
- Blood Test
- Genomic Test
- Others
On the basis of test type, the North America pancreatic cancer diagnostics market is segmented into imaging test, biopsy, blood test, genomic test, and others.
Cancer Stage
- Stage 0
- Stage I
- Stage II
- Stage III
- Stage IV
On the basis of cancer stage, the North America pancreatic cancer diagnostics market is segmented into stage 0, stage I, stage II, stage III and stage IV.
Tumor Type
- Exocrine Tumors
- Neuroendocrine Tumors
On the basis of tumor type, the North America pancreatic cancer diagnostics market is segmented into exocrine tumors and neuroendocrine tumors.
Product
- Instrument-Based Products
- Platform-Based Products
- Kits and Reagents
- Other Consumables
On the basis of product, the North America pancreatic cancer diagnostics market is segmented into instrument-based products, platform-based products, kits and reagents, and other consumables.
Application
- Screening
- Diagnostic And Predictive
- Prognostic
- Research
On the basis of application, the North America pancreatic cancer diagnostics market is segmented into screening, diagnostic and predictive, prognostic, and research.
Technology
- Fluorescent In Situ Hybridization
- Next Generation Sequencing
- Fluoroimmunoassay
- Comparative Genomic Hybridization
- Immunohistochemical
- Others
On the basis of technology, the North America pancreatic cancer diagnostics market is segmented into fluorescent in situ hybridization, next generation sequencing, fluoroimmunoassay, comparative genomic hybridization, immunohistochemical, and others.
End User
- Hospitals
- Diagnostic Centers
- Cancer Research Centers
- Academic Institutes
- Ambulatory Surgical Centers
- Others
On the basis of end user, the North America pancreatic cancer diagnostics market is segmented into hospitals, diagnostic centers, cancer research centers, academic institutes, ambulatory surgical centers, and others.
Distribution Channel
- Direct Tender
- Retail Sales
- Others
On the basis of distribution channel, the North America pancreatic cancer diagnostics market is segmented into direct tender, retail sales and others.
North America Pancreatic Cancer Diagnostics Market Country Analysis/Insights
North America pancreatic cancer diagnostics market is analyzed, and market size insights and trends are provided by the country, test type, cancer stages, tumor type, product, application, technology, end user and distribution channel as referenced above.
- In 2023, U.S. pancreatic cancer diagnostics market is expected to grow due to rise in prevalence and incidence of pancreatic cancer and increase in awareness about the pancreatic cancer diagnostics. These are the key contributing factors which is expected to boost the growth of the market in the country.
The country section of the report also provides individual market-impacting factors and changes in market regulation that impact the current and future trends of the market. Data points such as downstream and upstream value chain analysis, technical trends, porter's five forces analysis, and case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Pancreatic Cancer Diagnostics Market Share Analysis
North America pancreatic cancer diagnostics market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the company’s focus on the North America pancreatic cancer diagnostics market.
Some of the major players operating in the market are Siemens Healthcare Private Limited, Koninklijke Philips N.V., FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, DiaSource, Abbott, Agilent Technologies, Inc., Lee Biosolutions, Inc, MP BIOMEDICALS, Setia Scientific Solution, Boditech Med Inc., AccuBioTech Co., Ltd., Thermo Fisher Scientific, Creative Biolabs, Myriad Genetics, Inc., BD, CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Meridian Life Science, Inc., CTK Biotech, Inc., among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, INDUSTRY INSIGHTS
6 EPIDEMIOLOGY
7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROW IN PREVALENCE OF PANCREATIC CANCER
8.1.2 NOVEL TECHNOLOGICAL ADVANCEMENTS IN PANCREATIC DIAGNOSTICS
8.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
8.1.4 INCREASE IN AWARENESS REGARDING PANCREATIC CANCER
8.2 RESTRAINTS
8.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF PANCREATIC CANCER DIAGNOSTIC PRODUCTS
8.2.2 LATE DIAGNOSIS AND POOR PROGNOSIS OF PANCREATIC CANCER
8.3 OPPORTUNITIES
8.3.1 INCREASE IN DIAGNOSTIC PRODUCTS FOR PANCREATIC CANCER
8.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
8.3.3 GOVERNMENT INITIATIVES TOWARD PANCREATIC CANCER DIAGNOSTICS
8.4 CHALLENGES
8.4.1 INCREASED COST, SAFETY, AND CONVENIENCE ISSUES
8.4.2 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.2 MAGNETIC RESONANCE IMAGING (MRI)
9.2.2.1 MR CHOLANGIOPANCREATOGRAPHY
9.2.2.2 MR ANGIOGRAPHY (MRA)
9.2.3 ULTRASOUND
9.2.3.1 ABDOMINAL ULTRASOUND
9.2.3.2 ENDOSCOPIC ULTRASOUND (EUS)
9.2.4 CHOLANGIOPANCREATOGRAPHY
9.2.4.1 MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY (MRCP)
9.2.4.2 ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ERCP)
9.2.4.3 PRECUTANEOUS TRANSHEPTIC CHOLANGIOPANCREATOGRAPHY (PTC)
9.2.5 POSITRON EMISSION TOMOHRAPHY (PET)
9.2.6 OTHERS
9.3 BIOPSY
9.3.1 CT-GUIDED NEEDLE BIOPSY
9.3.2 FINE NEEDLE ASPIRATION (FNA)
9.3.3 CORE NEEDLE BIOPSY
9.3.4 OTHERS
9.4 BLOOD TEST
9.4.1 LIVER FUNCTION TEST
9.4.2 TUMOR MARKER
9.4.2.1 CA 19-9 BIOMARKER TEST
9.4.2.2 CARCINOEMBROYNIC ANTIGEN (CEA) TEST
9.4.2.3 CA 50 MARKER TEST
9.4.2.4 OTHERS
9.4.3 OTHERS
9.5 GENOMIC TEST
9.6 OTHERS
10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES
10.1 OVERVIEW
10.2 STAGE IV
10.3 STAGE III
10.4 STAGE II
10.4.1 STAGE IIA
10.4.2 STAGE IIB
10.5 STAGE I
10.5.1 STAGE IA
10.5.2 STAGE IB
10.6 STAGE 0
11 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
11.1 OVERVIEW
11.2 EXOCRINE TUMORS
11.2.1 INSTRUMENT-BASED PRODUCTS
11.2.2 PLATFORM-BASED PRODUCTS
11.2.3 KITS AND REAGENTS
11.2.4 OTHER CONSUMABLES
11.3 NEUROENDOCRINE TUMORS
11.3.1 INSTRUMENT-BASED PRODUCTS
11.3.2 PLATFORM-BASED PRODUCTS
11.3.3 KITS AND REAGENTS
11.3.4 OTHER CONSUMABLES
12 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT
12.1 OVERVIEW
12.2 INSTRUMENT-BASED PRODUCTS
12.2.1 IMAGING
12.2.2 BIOPSY
12.3 PLATFORM-BASED PRODUCTS
12.3.1 NEXT-GENERATION SEQUENCING
12.3.2 MICROARRAYS
12.3.3 PCR
12.3.4 OTHERS
12.4 KITS AND REAGENTS
12.4.1 CA19-9 PANCREATIC CANCER TEST KITS
12.4.1.1 ELISA TEST KITS
12.4.1.2 CASETTE TEST KITS
12.4.1.3 OTHERS
12.4.2 CEA PANCREATIC CANCER TEST KITS
12.4.2.1 ELISA TEST KITS
12.4.2.2 CASETTE TEST KITS
12.4.2.3 OTHERS
12.5 OTHER CONSUMABLES
13 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
13.1 OVERVIEW
13.2 FLUORESCENT IN SITU HYBRIDIZATION
13.3 NEXT GENERATION SEQUENCING
13.4 FLUORIMMUNOASSAY
13.5 COMPARATIVE GENOMIC HYBRIDIZATION
13.6 IMMUNOHISTOCHEMICAL
13.7 OTHERS
14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION
14.1 OVERVIEW
14.2 SCREENING
14.2.1 INSTRUMENT-BASED PRODUCTS
14.2.2 PLATFORM-BASED PRODUCTS
14.2.3 KITS AND REAGENTS
14.2.4 OTHER CONSUMABLES
14.3 DIAGNOSTIC AND PREDICTIVE
14.3.1 INSTRUMENT-BASED PRODUCTS
14.3.2 PLATFORM-BASED PRODUCTS
14.3.3 KITS AND REAGENTS
14.3.4 OTHER CONSUMABLES
14.4 PROGNOSTIC
14.4.1 INSTRUMENT-BASED PRODUCTS
14.4.2 PLATFORM-BASED PRODUCTS
14.4.3 KITS AND REAGENTS
14.4.4 OTHER CONSUMABLES
14.5 RESEARCH
14.5.1 INSTRUMENT-BASED PRODUCTS
14.5.2 PLATFORM-BASED PRODUCTS
14.5.3 KITS AND REAGENTS
14.5.4 OTHER CONSUMABLES
15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.3 DIAGNOSTIC CENTERS
15.4 CANCER RESEARCH CENTERS
15.5 ACADEMIC INSTITUTES
15.6 AMBULATORY SURGICAL CENTERS
15.7 OTHERS
16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION
17.1 NORTH AMERICA
17.1.1 U.S.
17.1.2 CANADA
17.1.3 MEXICO
18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
19 SWOT ANALYSIS
20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
20.1 CANON MEDICAL SYSTEMS CORPORATION
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENT
20.2 KONINKLIJKE PHILIPS N.V.
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 SIEMENS HEALTHCARE GMBH
20.3.1 COMPANY SNAPSHOT
20.3.2 REVENUE ANALYSIS
20.3.3 COMPANY SHARE ANALYSIS
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENT
20.4 GRAIL
20.4.1 COMPANY PROFILE
20.4.2 COMPANY SHARE ANALYSIS
20.4.3 PRODUCT PORTFOLIO
20.4.4 RECENT DEVELOPMENT
20.5 MYRIAD GENETICS, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 REVENUE ANALYSIS
20.5.3 COMPANY SHARE ANALYSIS
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENT
20.6 BD
20.6.1 COMPANY SNAPSHOT
20.6.2 REVENUE ANALYSIS
20.6.3 PRODUCT PORTFOLIO
20.6.4 RECENT DEVELOPMENT
20.7 BODITECH MED INC.
20.7.1 COMPANY PROFILE
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.8 ABBOTT (2022)
20.8.1 COMPANY SNAPSHOT
20.8.2 REVENUE ANALYSIS
20.8.3 PRODUCT PORTFOLIO
20.8.4 RECENT DEVELOPMENT
20.9 FUJIFILM HOLDINGS AMERICA CORPORATION
20.9.1 COMPANY SNAPSHOT
20.9.2 REVENUE ANALYSIS
20.9.3 PRODUCT PORTFOLIO
20.9.4 RECENT DEVELOPMENT
20.1 ACCUBIOTECH CO., LTD.
20.10.1 COMPANY PROFILE
20.10.2 PRODUCT PORTFOLIO
20.10.3 RECENT DEVELOPMENTS
20.11 AGILENT TECHNOLOGIES, INC.
20.11.1 COMPANY PROFILE
20.11.2 REVENUE ANALYSIS
20.11.3 PRODUCT PORTFOLIO
20.11.4 RECENT DEVELOPMENT
20.12 CREATIVE BIOLABS.
20.12.1 COMPANY PROFILE
20.12.2 PRODUCT PORTFOLIO
20.12.3 RECENT DEVELOPMENT
20.13 CTK BIOTECH, INC.
20.13.1 COMPANY PROFILE
20.13.2 PRODUCT PORTFOLIO
20.13.3 RECENT DEVELOPMENT
20.14 DIASOURCE
20.14.1 COMPANY SNAPSHOT
20.14.2 PRODUCT PORTFOLIO
20.14.3 RECENT DEVELOPMENT
20.15 LABORATORY CORPORATION OF AMERICA HOLDINGS
20.15.1 COMPANY SNAPSHOT
20.15.2 REVENUE ANALYSIS
20.15.3 PRODUCT PORTFOLIO
20.15.4 RECENT DEVELOPMENTS
20.16 LEE BIOSCIENCE
20.16.1 COMPANY SNAPSHOT
20.16.2 PRODUCT PORTFOLIO
20.16.3 RECENT DEVELOPMENT
20.17 MERIDIAN BIOSCIENCE INC.
20.17.1 COMPANY PROFILE
20.17.2 PRODUCT PORTFOLIO
20.17.3 RECENT DEVELOPMENT
20.18 MP BIOMEDICALS.
20.18.1 COMPANY PROFILE
20.18.2 PRODUCT PORTFOLIO
20.18.3 RECENT DEVELOPMENTS
20.19 QIAGEN
20.19.1 COMPANY SNAPSHOT
20.19.2 REVENUE ANALYSIS
20.19.3 PRODUCT PORTFOLIO
20.19.4 RECENT DEVELOPMENT
20.2 SETIA SCIENTIFIC SOLUTION
20.20.1 COMPANY PROFILE
20.20.2 PRODUCT PORTFOLIO
20.20.3 RECENT DEVELOPMENTS
20.21 THERMO FISHER SCIENTIFIC INC.
20.21.1 COMPANY SNAPSHOT
20.21.2 REVENUE ANALYSIS
20.21.3 PRODUCT PORTFOLIO
20.21.4 RECENT DEVELOPMENT
21 QUESTIONNAIRE
22 RELATED REPORTS
List of Table
TABLE 1 APPROVED DIAGNOSTICS OF PANCREATIC CANCER
TABLE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA GENOMIC TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA STAGE IV IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA STAGE III IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA STAGE 0 IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA OTHER CONSUMABLES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA FLUORESCENT IN SITU HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA NEXT GENERATION SEQUENCING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA FLUORIMMUNOASSAY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA COMPARATIVE GENOMIC HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA IMMUNOHISTOCHEMICAL IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA HOSPITALS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA DIAGNOSTIC CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA CANCER RESEARCH CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA ACADEMIC INSTITUTES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA DIRECT TENDER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA RETAIL SALES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.S. IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 U.S. MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 U.S. ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.S. CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 103 U.S. BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 U.S. TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 U.S. BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 107 U.S. STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 108 U.S. STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 109 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 110 U.S. EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 U.S. NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 112 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 113 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 U.S. KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 120 U.S. CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 121 U.S. CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 123 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 124 U.S. SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 U.S. DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 126 U.S. PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 127 U.S. RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 128 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 129 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 130 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 CANADA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 132 CANADA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 133 CANADA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 CANADA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 CANADA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 136 CANADA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 CANADA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 139 CANADA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 140 CANADA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 141 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 142 CANADA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 CANADA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 144 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 145 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 149 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 150 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 151 CANADA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 CANADA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 CANADA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 155 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 156 CANADA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 157 CANADA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 158 CANADA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 159 CANADA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 160 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 MEXICO IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 MEXICO MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 165 MEXICO ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 MEXICO CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 MEXICO BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 168 MEXICO TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 169 MEXICO BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 170 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 171 MEXICO STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 172 MEXICO STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 173 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 174 MEXICO EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 MEXICO NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 176 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 177 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 178 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 179 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 180 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 182 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 183 MEXICO KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 184 MEXICO CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 185 MEXICO CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 186 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 187 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 188 MEXICO SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 189 MEXICO DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 190 MEXICO PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 191 MEXICO RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 192 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF PANCREATIC CANCER AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2022
FIGURE 19 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2022
FIGURE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2022
FIGURE 27 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 30 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2022
FIGURE 31 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 34 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2022
FIGURE 35 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 39 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 43 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 44 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 46 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 47 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 48 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 49 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 50 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: CATEGORY (2023-2030)
FIGURE 51 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.