North America Multiple Hereditary Exostosis Market
Market Size in USD Million
CAGR :
% 
 USD
51.36 Million
USD
65.57 Million
2024
2032
USD
51.36 Million
USD
65.57 Million
2024
2032
| 2025 –2032 | |
| USD 51.36 Million | |
| USD 65.57 Million | |
|
|
|
|
North America Multiple Hereditary Exostosis Market Size
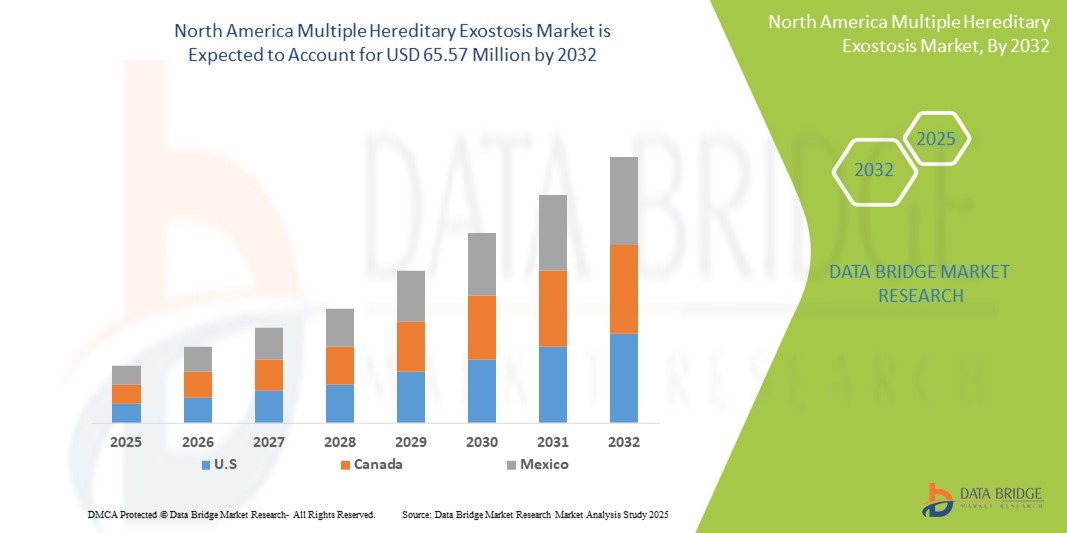
- The North America multiple hereditary exostosis market size was valued at USD 51.36 million in 2024 and is expected to reach USD 65.57 million by 2032, at a CAGR of 3.10% during the forecast period
- The market growth is largely driven by increased awareness, earlier diagnostic capabilities, and expanding genetic testing services, which have improved identification and clinical management of MHE across the region
- Furthermore, growing investments in orphan disease research, patient support initiatives, and novel treatment options such as gene therapies and targeted drug development are positioning MHE care as a vital part of rare disease healthcare frameworks. These synergistic developments are enhancing treatment pipelines and patient access, thereby significantly accelerating market growth
North America Multiple Hereditary Exostosis Market Analysis
- Multiple Hereditary Exostosis (MHE), a rare genetic disorder characterized by the development of multiple benign bone tumors, is gaining increasing attention in North America due to advances in diagnostic technologies, growing disease awareness, and the expansion of specialized treatment centers
- The rising demand for early diagnosis and tailored therapeutic approaches is primarily fueled by enhanced access to genetic testing, supportive healthcare policies, and increased focus on rare disease management by public and private sectors
- U.S. dominated the North America multiple hereditary exostosis market with the largest revenue share of 45.8% in 2024, driven by a strong healthcare infrastructure, active rare disease research programs, and the presence of leading biopharmaceutical companies engaged in orphan drug development and clinical trials
- Canada is expected to be the fastest growing country in the North America multiple hereditary exostosis market during the forecast period due to rising awareness initiatives, expanded rare disease registries, and growing access to specialized healthcare services
- The genetic tests segment dominated the North America multiple hereditary exostosis market with a market share of 49.2% in 2024, attributed to its essential role in early detection, family screening, and enabling individualized treatment plans
Report Scope and North America Multiple Hereditary Exostosis Market Segmentation
|
Attributes |
North America Multiple Hereditary Exostosis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Multiple Hereditary Exostosis Market Trends
“Increased Emphasis on Early Diagnosis and Precision Care”
- A significant and accelerating trend in the North America MHE market is the growing focus on early diagnosis and precision treatment through the use of advanced genetic screening, imaging, and individualized care strategies. These developments are enabling better disease identification and proactive clinical management for patients at various stages
- For instance, institutions such as Boston Children’s Hospital and the Mayo Clinic are leveraging next-generation sequencing technologies to identify EXT1 and EXT2 mutations in at-risk individuals, enabling early-stage detection and surveillance. In parallel, pediatric orthopedic clinics are adopting advanced imaging tools for more accurate monitoring of exostosis progression
- The integration of genetic insights into clinical decision-making supports tailored treatment plans, including the timing of surgical interventions and risk-based monitoring protocols. Digital health solutions, including patient registries and AI-powered data platforms, are also helping track long-term outcomes and refine clinical guidelines. Furthermore, early diagnosis initiatives are supported by advocacy organizations such as the MHE Research Foundation, which collaborates with researchers and clinicians to improve disease education and care
- The seamless combination of diagnostics, digital platforms, and personalized care planning is enabling healthcare providers to offer proactive treatment pathways, especially in pediatric cases where early intervention can prevent skeletal deformities and mobility issues
- This trend towards more targeted, data-driven, and patient-centric approaches is fundamentally reshaping expectations for MHE care and enhancing quality of life for affected individuals. Consequently, leading U.S. research institutions and biotech firms are exploring the potential of novel gene therapies and disease-modifying interventions
- The demand for precision-based MHE diagnosis and treatment models is growing rapidly across both academic medical centers and specialized pediatric clinics, as families and clinicians increasingly prioritize early detection, minimally invasive care, and long-term disease monitoring
North America Multiple Hereditary Exostosis Market Dynamics
Driver
“Rising Awareness and Expanding Access to Genetic Testing”
- The increasing public and clinical awareness of MHE, along with broader access to genetic testing and specialized diagnostic services, is a major driver accelerating the growth of the MHE market in North America
- For instance, initiatives by rare disease organizations such as the National Organization for Rare Disorders (NORD) and patient advocacy groups are promoting earlier diagnosis and facilitating more informed clinical decision-making. This is helping families seek genetic counseling and initiate early treatment interventions
- As genetic testing becomes more affordable and widely available, healthcare providers are better equipped to screen high-risk individuals and offer predictive insights. The expanding presence of pediatric orthopedic specialists and academic rare disease centers is further supporting comprehensive care strategies
- Furthermore, the integration of MHE care into multidisciplinary treatment models and institutional rare disease programs is encouraging collaborative research and innovation. The availability of family-based screening, detailed imaging protocols, and dedicated MHE clinics is fostering timely and effective care
- The convenience and accessibility of modern genetic platforms, along with growing acceptance among physicians and caregivers, are propelling adoption in both urban and underserved areas. This progress is contributing to earlier detection and improved clinical outcomes across the North American MHE landscape
Restraint/Challenge
“Limited Disease-Specific Therapies and Underdiagnosis”
- Despite increasing awareness, the MHE market in North America continues to face challenges such as the lack of disease-modifying therapies and frequent underdiagnosis due to the variable nature of symptom presentation, especially in early stages or in mild phenotypes
- For instance, many individuals with MHE may remain undiagnosed until skeletal deformities or complications emerge, resulting in delayed care. In addition, limited clinical exposure to rare diseases among general practitioners can hinder early detection and referral to specialists
- The absence of FDA-approved treatments that directly target the genetic basis of MHE constrains therapeutic development and results in a reliance on symptomatic and surgical management. While orthopedic surgeries can address deformities and relieve pain, they do not halt disease progression
- Moreover, reimbursement challenges, unequal access to genetic counseling, and disparities in healthcare infrastructure may restrict timely diagnosis and specialized care, particularly in rural or lower-income regions. These limitations can contribute to inconsistent treatment quality and long-term patient burden
- While public-private partnerships and patient advocacy groups are working to improve awareness and access, further efforts in standardizing care pathways, supporting clinical trials, and investing in gene-based therapies will be critical to overcoming existing challenges and ensuring sustainable market growth
North America Multiple Hereditary Exostosis Market Scope
The market is segmented on the basis of type, treatment, diagnosis, site, age group, and end user.
- By Type
On the basis of type, the North America multiple hereditary exostosis market is segmented into sessile and pedunculated. The sessile segment dominated the market with the largest market revenue share in 2024, driven by its higher clinical visibility and association with more severe skeletal complications. Sessile osteochondromas are typically broad-based and often found on major bones, which increases the such aslihood of early detection and surgical intervention. The segment also sees strong demand due to its correlation with mobility issues and deformities that require long-term orthopedic care.
The pedunculated segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by increasing diagnostic sensitivity and improved imaging capabilities. Pedunculated lesions, often less symptomatic initially, are being detected more frequently through proactive screening and genetic testing, especially in pediatric patients with a family history of MHE.
- By Treatment
On the basis of treatment, the North America multiple hereditary exostosis market is segmented into surgery, medication, and others. The surgery segment held the largest market revenue share in 2024 driven by its established role as the primary treatment modality for symptomatic lesions, deformities, and nerve compression. Surgical intervention is often necessary to restore mobility, relieve pain, and correct limb length discrepancies, particularly in growing children.
The medication segment is expected to witness the fastest CAGR from 2025 to 2032, driven by the growing use of pain management drugs, anti-inflammatories, and research into targeted molecular therapies. Although currently supportive in nature, medications are becoming an increasingly important component of non-surgical MHE care, especially for patients with mild symptoms or those ineligible for surgery.
- By Diagnosis
On the basis of diagnosis, the North America multiple hereditary exostosis market is segmented into X-ray, Computed Tomography (CT) Scan, Magnetic Resonance Imaging (MRI), Genetic Tests, and Others. The genetic tests segment dominated the MHE market with a market share of 49.2% in 2024, attributed to its essential role in early detection, family screening, and enabling individualized treatment plans. As genetic counseling becomes a regular part of MHE management, next-generation sequencing and EXT1/EXT2 mutation tests are gaining popularity for at-risk individuals and newly diagnosed pediatric cases.
The X-ray segment is expected to witness fastest growth from 2025 to 2032, driven by its continued use as a first-line, cost-effective diagnostic method in orthopedic and pediatric clinics. Despite being less advanced, X-rays remain widely accessible and serve as a standard initial screening tool for identifying bony outgrowths in symptomatic individuals.
- By Site
On the basis of site, the North America multiple hereditary exostosis market is segmented into legs, arms, shoulders, pelvis, fingers, and toes. The legs segment dominated the market with the largest market revenue share in 2024, driven by the high frequency of exostoses on the femur and tibia, which often result in gait disturbances and limb length discrepancies that require intervention.
The pelvis segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by growing awareness of exostoses in deeper skeletal areas. Pelvic lesions, though less visible externally, are increasingly diagnosed due to advancements in imaging technologies such as MRI and CT scans, and they often require complex surgical approaches due to proximity to nerves and internal structures.
- By Age Group
On the basis of age group, the North America multiple hereditary exostosis market is segmented into pediatric and adult. The pediatric segment dominated the market with the largest market revenue share in 2024, driven by the early onset nature of MHE, which typically manifests in childhood. Pediatric patients require consistent monitoring and early interventions to prevent long-term skeletal deformities, making this segment the largest consumer of orthopedic and diagnostic services.
The adult segment is anticipated to witness fastest growth from 2025 to 2032, supported by long-term care requirements, follow-up surgeries, and increased diagnosis of mild or delayed-onset cases. Adults with MHE may also require revision procedures and ongoing management of complications that arise over time.
- By End User
On the basis of end user, the North America multiple hereditary exostosis market is segmented into hospitals, specialty clinics, ambulatory surgical centers, and others. The hospitals segment held the largest market revenue share in 2024 driven by the comprehensive services provided in these settings, including diagnosis, imaging, orthopedic surgery, and genetic counseling. Hospitals are often the first point of care for pediatric referrals and complex cases requiring multidisciplinary treatment.
The specialty clinics segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by the emergence of dedicated rare disease and orthopedic clinics offering personalized care. These clinics provide coordinated treatment pathways for MHE patients, including access to genetic counseling, minimally invasive surgery, and long-term follow-up, making them increasingly preferred by families and caregivers seeking specialized expertise.
North America Multiple Hereditary Exostosis Market Regional Analysis
- U.S dominated the North America MHE market with the largest revenue share of 45.8% in 2024, driven by a strong healthcare infrastructure, active rare disease research programs, and the presence of leading biopharmaceutical companies engaged in orphan drug development and clinical trials
- Patients and healthcare providers in the U.S. prioritize early diagnosis and personalized treatment, supported by access to cutting-edge genetic testing, orthopedic expertise, and dedicated rare disease programs across leading pediatric institutions
- This strong market presence is further supported by active patient advocacy organizations, federal funding for rare disease research, and the integration of precision medicine approaches, making the U.S. a central hub for innovation and specialized MHE care in North America
U.S. Multiple Hereditary Exostosis Market Insight
The U.S. multiple hereditary exostosis market captured the largest revenue share of 87% in 2024 within North America, fueled by early genetic screening adoption, advanced pediatric orthopedic infrastructure, and growing public awareness of rare diseases. Leading hospitals and research institutions, such as Boston Children’s Hospital and Mayo Clinic, are pioneering personalized treatment approaches and multidisciplinary care models. Strong support from advocacy groups, coupled with ongoing clinical research into gene-targeted therapies and surgical advancements, is accelerating market expansion. Furthermore, the presence of established rare disease registries and federal initiatives is significantly contributing to the development of a comprehensive care ecosystem for MHE.
Canada Multiple Hereditary Exostosis Market Insight
The Canada multiple hereditary exostosis market is projected to expand at a stable CAGR throughout the forecast period, driven by increasing access to genetic counseling and the expansion of rare disease programs. National health initiatives are promoting awareness and early diagnosis of hereditary skeletal conditions. Pediatric hospitals across major cities, including Toronto and Vancouver, are increasingly adopting integrated care models, enhancing diagnosis and long-term management. In addition, the emphasis on universal healthcare and equity in rare disease treatment access is supporting the gradual uptake of specialized MHE services across provinces.
Mexico Multiple Hereditary Exostosis Market Insight
The Mexico multiple hereditary exostosis market is anticipated to grow at a moderate CAGR during the forecast period, supported by the gradual modernization of the country’s healthcare infrastructure and rising awareness of pediatric bone disorders. Though access to advanced diagnostics remains limited in rural areas, urban healthcare centers are increasingly incorporating imaging and genetic services. Collaborations with international research bodies and advocacy organizations are helping drive education and diagnosis initiatives. As government-backed efforts expand rare disease support and diagnostic capabilities, Mexico is positioned to see increased identification and treatment of MHE cases.
North America Multiple Hereditary Exostosis Market Share
The North America multiple hereditary exostosis industry is primarily led by well-established companies, including:
- Illumina, Inc. (U.S.)
- Medtronic (Ireland)
- Zimmer Biomet (U.S.)
- Stryker (U.S.)
- Siemens Healthineers AG (Germany)
- GE HealthCare (U.S.)
- Canon Medical Systems Corporation (Japan)
- Esaote S.p.A. (Italy)
- Thermo Fisher Scientific Inc. (U.S.)
- F. Hoffmann-La Roche AG (Switzerland)
- Koninklijke Philips N.V. (Netherlands)
- B. Braun SE (Germany)
- Agilent Technologies, Inc. (U.S.)
- PerkinElmer (U.S.)
- FUJIFILM Corporation (Japan)
- Beijing Genomics Institute (BGI Genomics) (China)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- Jiangsu Trautec Medical Technology Co., Ltd. (China)
- Toshiba Corporation (Japan)
- Takara Bio Inc. (Japan)
What are the Recent Developments in North America Multiple Hereditary Exostosis Market?
- In May 2024, the U.S.-based nonprofit MHE Research Foundation launched a new national awareness and education campaign across pediatric hospitals, aimed at promoting early diagnosis of Multiple Hereditary Exostosis through genetic screening and orthopedic evaluation. This initiative highlights the growing emphasis on early intervention and coordinated care in rare skeletal disorders and aims to improve long-term outcomes by supporting timely and accurate diagnosis for at-risk children
- In April 2024, Children’s Hospital of Philadelphia (CHOP) announced the expansion of its Orthopedic Genetics Program, which includes enhanced diagnostic pathways and personalized treatment planning for MHE patients. The program integrates genetic testing with surgical consultation and long-term orthopedic monitoring, positioning CHOP as a leader in multidisciplinary care for hereditary skeletal conditions
- In March 2024, Mayo Clinic initiated a clinical research study focused on understanding long-term complications and surgical outcomes in adults previously diagnosed with MHE in childhood. The study aims to generate evidence-based guidelines for lifelong orthopedic monitoring and inform future therapeutic advancements for skeletal tumor prevention and management
- In February 2024, Canadian-based SickKids Hospital introduced a rare disease genetic panel that includes EXT1 and EXT2 mutation screening for early MHE detection. This addition to the hospital's pediatric genetic services demonstrates growing investment in precision medicine across Canada and a shift toward earlier, more targeted interventions for hereditary bone disorders
- In January 2024, the National Institutes of Health (NIH) provided funding to support the development of a centralized North American MHE Patient Registry, aimed at improving clinical trial recruitment, tracking disease progression, and fostering cross-border collaboration in research. This initiative reflects the region’s strategic focus on data-driven advancements and collaborative approaches to rare disease treatment and care innovation
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 PIPELINE ANALYSIS - OBSERVATORY DATA
4.4 EPIDEMIOLOGY
5 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: REGULATIONS
5.1 REGULATION IN UNITED STATES: U.S. FOOD AND DRUG ADMINISTRATION (FDA)
5.1.1 REGULATION IN EUROPE: EUROPEAN MEDICINES AGENCY (EMA)
5.2 REGULATION IN ASIA-PACIFIC (JAPAN): PHARMACEUTICAL AND MEDICAL DEVICES AGENCY (PMDA)
5.3 MEDICAL DEVICE STANDARDS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF GENETIC DISORDERS
6.1.2 GROWING PEDIATRIC POPULATION
6.1.3 DEVELOPMENT OF NOVEL THERAPIES
6.2 RESTRAINTS
6.2.1 HIGH COST OF ADVANCED THERAPIES
6.2.2 LIMITED AVAILABILITY OF THERAPIES
6.3 OPPORTUNITIES
6.3.1 INCREASE IN PATIENT CARE AND SUPPORT SYSTEMS
6.3.2 INCREASE IN THE NUMBER OF COLLABORATIONS AND PARTNERSHIPS
6.4 CHALLENGES
6.4.1 LIMITED AWARENESS ABOUT THE DISORDER
6.4.2 LACK OF DRUG APPROVALS ASSOCIATED WITH THE DISORDER
7 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE
7.1 OVERVIEW
7.2 SESSILE
7.3 PEDUNCULATED
8 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT
8.1 OVERVIEW
8.2 SURGERY
8.2.1 REMOVE THE TUMOR
8.2.2 LENGTHEN LIMBS
8.3 MEDICATION
8.3.1 HOSPITAL PHARMACIES
8.3.2 DRUGS STORES AND RETAIL PHARMACIES
8.3.3 ONLINE PHARMACIES
8.4 OTHERS
9 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS
9.1 OVERVIEW
9.2 X-RAY
9.2.1 SESSILE
9.2.2 PEDUNCULATED
9.3 COMPUTED TOMOGRAPHY (CT) SCAN
9.3.1 SESSILE
9.3.2 PEDUNCULATED
9.4 MAGNETIC RESONANCE IMAGING (MRI)
9.4.1 SESSILE
9.4.2 PEDUNCULATED
9.5 GENETIC TESTS
9.5.1 SESSILE
9.5.2 PEDUNCULATED
9.6 OTHERS
9.6.1 SESSILE
9.6.2 PEDUNCULATED
10 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE
10.1 OVERVIEW
10.2 LEGS
10.3 ARMS
10.4 SHOULDERS
10.5 PELVIS
10.6 FINGERS
10.7 TOES
11 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
12 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.2.1 PRIVATE
12.2.2 GOVERNMENT
12.3 SPECIALTY CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 NORTH AMERICA MULTIPLE HEREDITY EXOSTOSIS MARKET, BY REGION
13.1 NORTH AMERICA
13.1.1 U.S
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 BAYERS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT UPDATES
16.2 HALEON GROUP OF COMPANIES
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 BASF
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT UPDATES
16.4 VIATRIS INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT UPDATES
16.5 ACTIZAPHARMA
16.5.1 COMPANY SNAPSHOT
16.5.2 PRODUCT PORTFOLIO
16.5.3 RECENT UPDATES
16.6 ADVACARE PHARMA
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT UPDATES
16.7 AUROBINDO PHARMA
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT TABLETS
16.8 HALEON GROUP OF COMPANIES
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT UPDATES
16.9 IPSEN BIOPHARMACEUTICALS, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT UPDATES
16.1 MALLINCKRODT
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT UPDATES
16.11 TEVA PHARMACEUTICAL INDUSTRIES LTD.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT UPDATES
16.12 TAJ PHARMACEUTICALS LIMITED
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT UPDATES
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 PIPELINE ANALYSIS - OBSERVATORY DATA
TABLE 2 PIPELINE ANALYSIS - INTERVENTIONAL DATA
TABLE 3 SALES DATA - 2024
TABLE 4 SALES DATA - 2023
TABLE 5 SALES DATA - 2022
TABLE 6 SALES DATA - 2021
TABLE 7 SALES DATA - 2020
TABLE 8 SALES DATA - 2019
TABLE 9 SALES DATA - 2018
TABLE 10 COST OF PALOVAROTENE
TABLE 11 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 12 NORTH AMERICA SESSILE IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 13 NORTH AMERICA PEDUNCULATED IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 14 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 15 NORTH AMERICA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 16 NORTH AMERICA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 17 NORTH AMERICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 18 NORTH AMERICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 19 NORTH AMERICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 20 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 21 NORTH AMERICA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 22 NORTH AMERICA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 23 NORTH AMERICA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 24 NORTH AMERICA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 25 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 26 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 27 NORTH AMERICA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 28 NORTH AMERICA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 29 NORTH AMERICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 30 NORTH AMERICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 31 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 32 NORTH AMERICA LEGS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 33 NORTH AMERICA ARMS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 34 NORTH AMERICA SHOULDERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 35 NORTH AMERICA PELVIS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 36 NORTH AMERICA FINGERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 37 NORTH AMERICA TOES IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 38 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 39 NORTH AMERICA PEDIATRIC IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 40 NORTH AMERICA ADULT IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 41 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 42 NORTH AMERICA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 43 NORTH AMERICA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 44 NORTH AMERICA SPECIALTY CLINICS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 45 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 46 NORTH AMERICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 47 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY COUNTRY, 2022-2031 (USD THOUSAND)
TABLE 48 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 49 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 50 NORTH AMERICA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 51 NORTH AMERICA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 52 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 53 NORTH AMERICA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 54 NORTH AMERICA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 55 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 56 NORTH AMERICA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 57 NORTH AMERICA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 58 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 59 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 60 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 61 NORTH AMERICA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 62 U.S. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 63 U.S. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 64 U.S. SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 65 U.S. MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 66 U.S. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 67 U.S. X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 68 U.S. COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 69 U.S. MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 70 U.S. GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 71 U.S. OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 72 U.S. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 73 U.S. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 74 U.S. MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 75 U.S. HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 76 CANADA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 77 CANADA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 78 CANADA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 79 CANADA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 80 CANADA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 81 CANADA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 82 CANADA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 83 CANADA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 84 CANADA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 85 CANADA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 86 CANADA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 87 CANADA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 88 CANADA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 89 CANADA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 90 MEXICO MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 91 MEXICO MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 92 MEXICO SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 93 MEXICO MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 94 MEXICO MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 95 MEXICO X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 96 MEXICO COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 97 MEXICO MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 98 MEXICO GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 99 MEXICO OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 100 MEXICO MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 101 MEXICO MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 102 MEXICO MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 103 MEXICO HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
List of Figure
FIGURE 1 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 PRODUCT LIFELINE CURVE
FIGURE 9 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 10 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 12 EXECUTIVE SUMMARY
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 TWO SEGMENTS COMPRISE THE NORTH AMERICA MULTIPLE HEREDITY EXOSTOSIS MARKET, BY TYPE
FIGURE 15 RISING PREVALENCE OF GENETIC DISORDERS IS DRIVING THE GROWTH OF THE NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET FROM 2024 TO 2031
FIGURE 16 THE TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET IN 2024 AND 2031
FIGURE 17 MARKET OVERVIEW
FIGURE 18 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2023
FIGURE 19 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2024-2031 (USD THOUSAND)
FIGURE 20 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, CAGR (2024-2031)
FIGURE 21 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2023
FIGURE 23 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2024-2031 (USD THOUSAND)
FIGURE 24 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, CAGR (2024-2031)
FIGURE 25 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 26 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2023
FIGURE 27 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2024-2031 (USD THOUSAND)
FIGURE 28 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, CAGR (2024-2031)
FIGURE 29 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, LIFELINE CURVE
FIGURE 30 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2023
FIGURE 31 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2024-2031 (USD THOUSAND)
FIGURE 32 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, CAGR (2024-2031)
FIGURE 33 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, LIFELINE CURVE
FIGURE 34 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2023
FIGURE 35 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2024-2031 (USD THOUSAND)
FIGURE 36 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, CAGR (2024-2031)
FIGURE 37 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 38 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2023
FIGURE 39 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2024-2031 (USD THOUSAND)
FIGURE 40 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, CAGR (2024-2031)
FIGURE 41 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 NORTH AMERICA MULTIPLE HEREDITY EXOSTOSIS MARKET: SNAPSHOT (2023)
FIGURE 43 NORTH AMERICA MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY SHARE 2023 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.