North America Melanoma Cancer Diagnostics Market
Market Size in USD Million
CAGR :
% 
 USD
1,964.80 Million
USD
3,466.02 Million
2022
2030
USD
1,964.80 Million
USD
3,466.02 Million
2022
2030
| 2023 –2030 | |
| USD 1,964.80 Million | |
| USD 3,466.02 Million | |
|
|
|
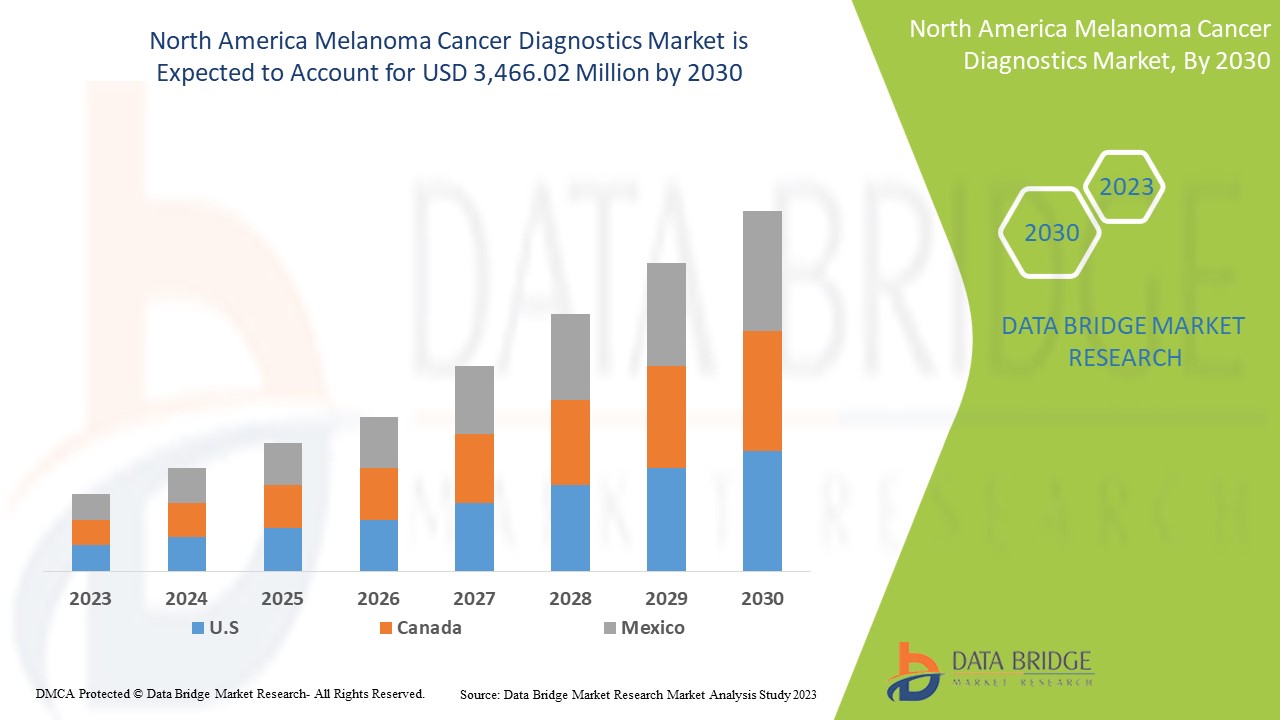
North America Melanoma Cancer Diagnostics Market Analysis and Insights
The increased demand for non-invasive testing methods globally has enhanced the market's demand. The rising healthcare expenditure for better health services is also contributing to the growth of the market. The major market players are highly focusing on various faster diagnostics during this crucial period. In addition, government initiatives for melanoma cancer diagnostics are also contributing to the rising demand for the melanoma cancer diagnostics market.
The increasing healthcare expenditure strategic initiatives by market players are giving opportunities to the market. However, the lack of skilled and certified professionals and the high cost of diagnostics procedures for melanoma cancers in emerging economies are key challenges to market growth.
North America melanoma cancer diagnostics market is expected to gain market growth in the forecast period of 2023 to 2030. Data Bridge Market Research analyses that the market is growing with a CAGR of 7.4% in the forecast period of 2023 to 2030 and is expected to reach USD 3,466.02 million by 2030 from USD 1,964.80 million in 2022.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customisable to 2020-1015) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Product Type (Instruments, Consumables and Accessories, and Others), Test Type (Biomarkers Test, Imaging Test, Biopsy, Fluorescent In Situ Hybridization (FISH) Tests, Comparative Genomic Hybridization (CGH) tests, Immunohistochemical (IHC) tests, and Others), End User (Hospitals, Associated Labs, Independent Diagnostic Laboratories, Diagnostic Imaging Centers, Cancer Research Institutes and Others), Distribution channel (Direct Tender and Retail Sales) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
Nanostring, Thermo Fisher Scientific Inc., Quest Diagnostics Incorporated, Agilent Technologies, Inc, QIAGEN, Inivata Ltd, F. Hoffman-La Roche Ltd, Abbott, AMLo Biosciences Limited, Myriad genetics Genetics Inc, Castle Biosciences, DermTech, Michael Diagnostics Ltd, Damae Medical, Skin Analytics, DermLite, DermaSensor, Skyline Dx, Neracare GmbH, VERISKIN INC., Illumina Inc, and bioMerieux SA, among others |
Market Definition
Melanoma cancer diagnostics are known as the process of identifying melanoma cancer by studying skin cells and molecules. These melanoma cancer diagnostics are used as a strategy to research, analyse and diagnose certain cells or molecules with the help of various tests performed in the laboratory. It is specially used for the measurement of a specific biomarker or identifying the biomarker in the skin cells. A melanoma cancer diagnosis is used with the aim of providing more efficient testing and faster diagnostics.
Melanoma cancer diagnostics help doctors find out the cancer stages to effectively treat patients at various stages. Furthermore, with the potential for clinical practice, several tests are used to give additional support to boost efficiency in melanoma cancer diagnosis, and the presence of major market players also contributes to the market growth.
North America Melanoma Cancer Diagnostics Market Dynamics
Drivers
-
Rising demand for early melanoma cancer diagnosis
A potentially fatal cancer, melanomas are most frequently found on the skin. The melanoma incidence has increased significantly on a North America scale. The incidence is highest among populations with fair skin and at lower latitudes. It is one of the cancers with the highest average number of years lost to disease per death. Melanoma is a severe personal and economic burden due to increased incidence and mortality. Various high-risk areas have employed preventative measures with varying degrees of success. The genesis of disease and risk factors must be better understood through research initiatives.
-
Rising preference for preventive health check-ups
Preventive health check-ups are preventive actions performed for the initial detection of melanoma cancer disease. Also, a rising preference for preventive health check-ups provides a safeguard against likely exposure to any disease in the future.
Awareness to promote screening is the most important component of melanoma cancer prevention. The check-up is comprised of the identification of cancer and examinations of risk factors to limit loss at an early stage.
Opportunities
-
Increasing healthcare expenditure for melanoma cancer treatment
Growing healthcare infrastructure is an opportunity for the market because if investment in healthcare increases, more people are getting aware of cancer disease and diagnose their health for precaution and cure.
Increasing healthcare expenditure for cancer treatment also helps the patient to take hassle-free advanced treatment for taking better diagnosis and treatment for fast recovery. The spending on health is made up of the combination of out-of-pocket payments (people paying for their own care), government expenditure, and sources, including health insurance and activities by non-governmental organizations. Due to this, increasing healthcare expenditure for cancer treatment is acting as an opportunity for growing the demand of the market.
-
Strategic initiatives by major players
Increasing rates of various types of disease and their severity are widely seen among people globally. The dramatic rise in research quality and increasing research opportunities is because of various strategic initiatives the market players take. They are taking initiatives such as product launch, collaborations, mergers, acquisitions, and many more over the years and is expected to lead and create more opportunities in the market. For instance, Evonik invested in the short-term growth of its specialty melanoma cancer diagnostics production at its Hanau and Dossenheim locations in Germany, which supplied two of the four melanoma cancer diagnostics for the Pfizer/BioNTech vaccine. According to Spencer, the first batches were delivered to BioNTech in April 2021, months ahead of schedule.
Restraints/Challenges
- Strict regulations and standards for the approval and commercialization of melanoma cancer diagnostic products
The stringent regulations for the commercialization of any product in the market are proving to be a big challenge for the manufacturers of cancer diagnostic products globally that have their own regulations and a different body for the regulatory procedures.
Manufacturers' approval for commercializing product commercializing into the market. Due to this, in the North America region, stringent regulatory policies are expected to hinder the development of the cancer diagnostic market.
The regulatory requirement for approvals of marketing or CE certification and application of laws and regulations could lead to making major changes in business or paying penalties, including the potential loss of business licenses. The resources and costs required to comply with these laws, rules, and regulations are quite high. Different manufacturing challenges for lipid nanoparticle production
Post-COVID-19 Impact on the North America Melanoma Cancer Diagnostics Market
COVID-19 has positively affected the market. As the demand for diagnostics increased, preventive health check-ups were in high demand. Thus COVID-19 affected the melanoma cancer diagnostics market positively.
Recent Development
- In October 2022, Quest Diagnostics Incorporated announced that the company has collaborated with Decode health to get biomarker-based data that can help reduce the time and cost of developing novel diagnostic tests and drug targets for different types of cancer. This will help the company to find innovative paths in the field of R&D and increases the North America presence of the company in the market
- In May 2022, Myriad Genetics, Inc. announced the expansion of its strategic partnership with Intermountain Precision Genomics, a service of Intermountain Healthcare, to add a new liquid biopsy therapy selection test to the company's growing oncology portfolio. This results in growing liquid biopsy space for the precise tumor test
North America Melanoma Cancer Diagnostics Market Scope
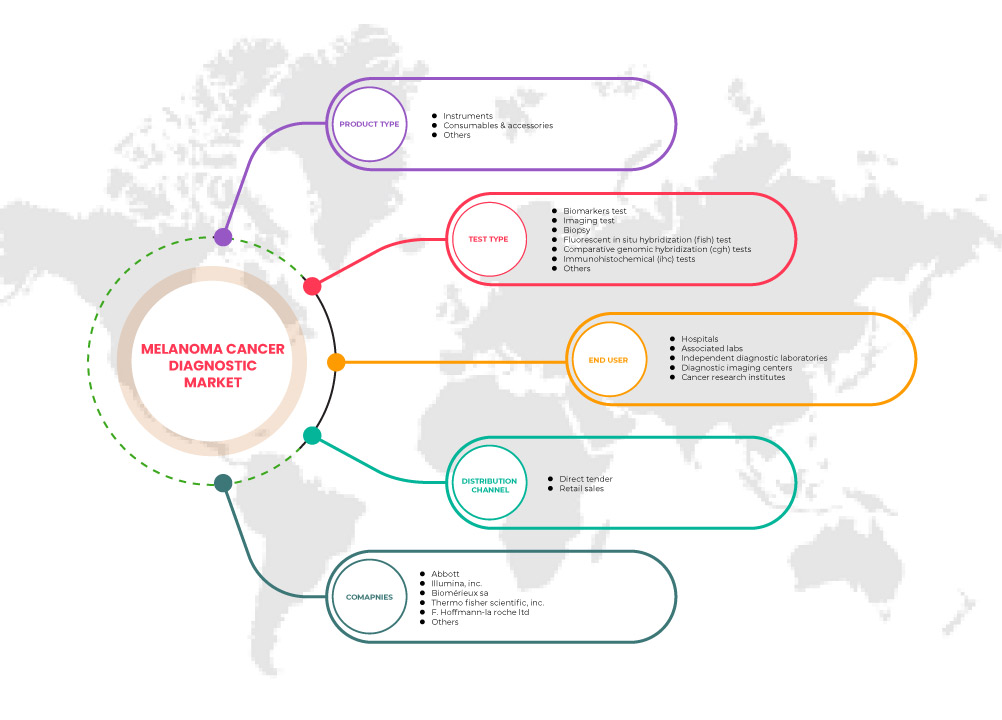
The North America melanoma cancer diagnostics market is segmented into product type, test type, end user, and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Product Type
- Instruments
- Consumables & Accessories
- Others
On the basis of type, the North America melanoma cancer diagnostics market is segmented into instruments, consumables & accessories, and others.
Test Type
- Biopsy
- Imaging test
- Immunohistochemical (IHC) tests
- Biomarkers test
- Fluorescent In Situ Hybridization (FISH) tests
- Comparative Genomic Hybridization (CGH) tests
- Others
Based on test type, the North America melanoma cancer diagnostics market is segmented into biomarkers test, imaging test, biopsy, Fluorescent In Situ Hybridization (FISH) tests, Comparative Genomic Hybridization (CGH) tests, Immunohistochemical (IHC) tests, and others.
End User
- Hospitals
- Associated labs
- Independent diagnostic laboratories
- Diagnostic imaging centers
- Cancer research institutes
- Others
Based on end user, the North America melanoma cancer diagnostics market is segmented into hospitals, associated labs, independent diagnostic laboratories, diagnostic imaging centers, cancer research institutes, and others.
Distribution Channel
- Direct Tender
- Retail Sales
- Others
Based on the distribution channel, the North America melanoma cancer diagnostics market is segmented into direct tender, retail sales, and others.
Melanoma Cancer Diagnostics Market Regional Analysis/Insights
The North America melanoma cancer market is analysed, and market size insights and trends are provided by product type, test type, end user, and distribution channel.
North America melanoma cancer market comprise of the countries the U.S., Canada, and Mexico.
The U.S. is expected to dominate the North America melanoma cancer diagnostics market due to growing demand for quality healthcare and rising demand for non-invasive testing methods.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of North America brands and their challenges faced due to high competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Melanoma Cancer Diagnostics Market Share Analysis
The North America melanoma cancer diagnostics market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on the North America melanoma cancer diagnostics market.
Some of the major players operating in the North America melanoma cancer diagnostics market are Nanostring, Thermo Fisher Scientific Inc., Quest Diagnostics Incorporated, Agilent Technologies, Inc, QIAGEN, Inivata Ltd, F. Hoffman-La Roche Ltd, Abbott, AMLo Biosciences Limited, Myriad genetics Genetics Inc, Castle Biosciences, DermTech, Michael Diagnostics Ltd, Damae Medical, Skin Analytics, DermLite, DermaSensor, Skyline Dx, Neracare GmbH, VERISKIN INC., Illumina Inc, and bioMerieux SA, among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL'S MODEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 INDUSTRY INSIGHTS
6.1 DEMOGRAPHIC TRENDS
6.2 KEY PRICING STRATEGIES
6.2.1 PRODUCT INNOVATION
6.2.2 CONSUMER AWARNESS
6.2.3 A VAST NETWORK OF DISTRIBUTION
6.2.4 PARTNERSHIP WITH POPULAR BRANDS BY MAJOR PLAYERS
6.2.5 OTHERS
6.3 KEY PATIENT ENROLLMENT STRATEGIES
6.3.1 IDENTIFICATION OF CUSTOMERS NEED FOR INNOVATIVE DIAGOSTIC PRODUCTS
6.3.2 INCREASING SPECIFIC TACTICS FOR EVERY STEP
6.3.3 EDUCATE AND COMMUNICATE
6.3.4 IMPROVING DIAGNOSIS SEEKING RATE
6.4 INTERVIEWS WITH MANUFACTURING COMPANIES
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING PREVALENCE OF MELANOMA CANCER
7.1.2 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.1.3 NOVEL TECHNOLOGIES IN MELANOMA CANCER DIAGNOSTICS
7.1.4 INCREASING AWARENESS REGARDING MELANOMA CANCER
7.2 RESTRAINTS
7.2.1 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7.2.2 HIGH COST OF DIAGNOSTICS PROCEDURE FOR MELANOMA CANCERS
7.3 OPPORTUNITIES
7.3.1 INCREASING HEALTHCARE EXPENDITURE FOR MELANOMA CANCER TREATMENT
7.3.2 GOVERNMENT INITIATIVES TOWARD MELANOMA CANCER DIAGNOSTICS
7.3.3 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
7.3.4 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE
7.4 CHALLENGES
7.4.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF MELANOMA CANCER DIAGNOSTIC PRODUCTS
7.4.2 RADIATION RISKS FROM IMAGING TESTS
8 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 INSTRUMENTS
8.2.1 IMAGING INSTRUMENTS
8.2.1.1 ULTRASOUND SYSTEMS
8.2.1.2 MRI SYSTEMS
8.2.1.3 CT SYSTEMS
8.2.1.4 OTHERS
8.2.2 BIOPSY INSTRUMENTS
8.2.3 PATHOLOGY-BASED INSTRUMENTS
8.2.3.1 PCR INSTRUMENTS
8.2.3.2 CELL PROCESSORS
8.2.3.3 SLIDE STAINING SYSTEMS
8.2.3.4 TISSUE PROCESSING SYSTEMS
8.2.3.5 OTHER PATHOLOGY-BASED INSTRUMENTS
8.3 CONSUMABLES & ACCESSORIES
8.3.1 KITS
8.3.1.1 PCR KITS
8.3.1.2 NUCLEIC ACID ISOLATION KITS
8.3.1.3 DNA POLYMERASE KITS
8.3.1.4 OTHERS
8.3.2 PROBES
8.3.2.1 Q FISH
8.3.2.2 FLOW FISH
8.3.2.3 OTHERS
8.3.3 REAGENTS
8.3.3.1 ASSAYS
8.3.3.2 BUFFERS
8.3.3.3 PRIMERS
8.3.3.4 OTHERS
8.3.4 OTHER CONSUMABLES
8.4 OTHERS
9 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 ULTRASOUND
9.2.2 MRI
9.2.3 CHEST X-RAY
9.2.4 LYMPHOSCINTIGRAPHY
9.2.5 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.6 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
9.2.7 OTHERS
9.3 BIOPSY
9.3.1 OPTICAL BIOPSY
9.3.2 EXCISIONAL BIOPSY
9.3.3 INCISIONAL BIOPSY
9.3.4 SHAVE BIOPSY
9.3.5 PUNCH BIOPSY
9.3.6 OTHERS
9.4 IMMUNOHISTOCHEMICAL (IHC) TESTS
9.4.1 S100 PROTEIN FAMILY BIOPSY
9.4.2 MELAN-A
9.4.3 PMEL/PMEL17/SILV/GP100
9.4.4 TYROSINASE
9.4.5 MITF
9.4.6 SM5-1
9.4.7 CSPG4/HMW-MAA
9.5 BIOMARKER TEST
9.5.1 BRAF MUTATION TEST
9.5.2 NRAS MUTATION TEST
9.5.3 CKIT TEST
9.5.4 OTHERS
9.6 FLUORESCENT IN SITU HYBRIDIZATION (FISH) TESTS
9.7 COMPARATIVE GENOMIC HYBRIDIZATION (CGH) TESTS
9.8 OTHERS
10 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 ASSOCIATED LABS
10.4 DIAGNOSTIC IMAGING CENTERS
10.5 INDEPENDENT DIAGNOSTIC LABORATORIES
10.6 CANCER RESEARCH INSTITUTES
10.7 OTHERS
11 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 RETAIL SALES
12 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 ABBOTT
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 ILLUMINA, INC.
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 BIOMÉRIEUX SA
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENTS
15.4 THERMO FISHER SCIENTIFIC INC.
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 F. HOFFMANN-LA ROCHE LTD.
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 AGILENT TECHNOLOGIES, INC.
15.6.1 COMPANY PROFILE
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AMLO BIOSCIENCES LIMITED
15.7.1 COMPANY PROFILE
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 CASTLE BIOSCIENCES INC
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 DAMAE MEDICAL
15.9.1 COMPANY PROFILE
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT DEVELOPMENT
15.1 DERMLITE.
15.10.1 COMPANY PROFILE
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENTS
15.11 DERMASENSOR
15.11.1 COMPANY PROFILE
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 DERMTECH
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 INIVATA LTD.
15.13.1 COMPANY PROFILE
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 MICHAEL DIAGNOSTICS LTD
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 MYRIAD GENETICS, INC.
15.15.1 COMPANY SNAPSHOT
15.15.2 REVENUE ANALYSIS
15.15.3 PRODUCT PORTFOLIO
15.15.4 RECENT DEVELOPMENT
15.16 NANOSTRING
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 NERACARE GMBH
15.17.1 COMPANY PROFILE
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 SKIN ANALYTICS
15.18.1 COMPANY PROFILE
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
15.19 SKYLINEDX
15.19.1 COMPANY PROFILE
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENTS
15.2 VERISKIN INC.
15.20.1 COMPANY PROFILE
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENTS
15.21 QIAGEN
15.21.1 COMPANY SNAPSHOT
15.21.2 REVENUE ANALYSIS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
15.22 QUEST DIAGNOSTICS INCORPORATED (2022)
15.22.1 COMPANY SNAPSHOT
15.22.2 REVENUE ANALYSIS
15.22.3 PRODUCT PORTFOLIO
15.22.4 RECENT DEVELOPMENTS
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 2 NORTH AMERICA INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA IMAGING INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA CONSUMERS & ACCESSORIES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CONSUMABLES & ACCESSORIES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA KITS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA PROBES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA REAGENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA OTHERS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA IMAGING TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA IMAGING TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA BIOPSY IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA BIOPSY IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA IMMUNOHISTOCHEMICAL (IHC) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA IMMUNOHISTOCHEMICAL (IHC) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA BIOMARKERS TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA BIOMARKERS TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA FLUORESCENT IN SITU HYBRIDIZATION (FISH) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA COMPARATIVE GENOMIC HYBRIDIZATION (CGH) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA OTHERS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA HOSPITALS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA ASSOCIATED LABS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA DIAGNOSTIC IMAGING CENTERS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA INDEPENDENT DIAGNOSTIC LABORATORIES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA CANCER RESEARCH INSTITUTES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA OTHERS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA DIRECT TENDER IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA RETAIL SALES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA IMAGING INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA CONSUMABLES & ACCESSORIES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA KITS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA REAGENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA PROBES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA BIOMARKERS TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA IMAGING TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA BIOPSY IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA IMMUNOHISTOCHEMICAL (IHC) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 50 U.S. MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 U.S. INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 52 U.S. PATHOLOGY-BASED INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 53 U.S. IMAGING INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 54 U.S. CONSUMABLES & ACCESSORIES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 55 U.S. KITS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 56 U.S. REAGENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 57 U.S. PROBES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 58 U.S. MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 59 U.S. BIOMARKERS TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 60 U.S. IMAGING TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 61 U.S. BIOPSY IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 62 U.S. IMMUNOHISTOCHEMICAL (IHC) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 63 U.S. MELANOMA CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 64 U.S. MELANOMA CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 65 CANADA MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 66 CANADA INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 67 CANADA PATHOLOGY-BASED INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 68 CANADA IMAGING INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 69 CANADA CONSUMABLES & ACCESSORIES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 70 CANADA KITS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 71 CANADA REAGENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 72 CANADA PROBES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 73 CANADA MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 CANADA BIOMARKERS TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 75 CANADA IMAGING TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 76 CANADA BIOPSY IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 77 CANADA IMMUNOHISTOCHEMICAL (IHC) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 78 CANADA MELANOMA CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 79 CANADA MELANOMA CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 80 MEXICO MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 MEXICO INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 MEXICO PATHOLOGY-BASED INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 MEXICO IMAGING INSTRUMENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 MEXICO CONSUMABLES & ACCESSORIES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 85 MEXICO KITS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 86 MEXICO REAGENTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 87 MEXICO PROBES IN MELANOMA CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 88 MEXICO MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 89 MEXICO BIOMARKERS TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 90 MEXICO IMAGING TEST IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 91 MEXICO BIOPSY IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 92 MEXICO IMMUNOHISTOCHEMICAL (IHC) TESTS IN MELANOMA CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 93 MEXICO MELANOMA CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 94 MEXICO MELANOMA CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE GROWING PREVALENCE OF MELANOMA CANCER AND THE INCREASING AWARENESS REGARDING MELANOMA CANCER ARE EXPECTED TO DRIVE THE NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET GROWTH IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 INSTRUMENTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 ESTIMATED PREVALENCE OF MELANOMA CANCER IN 2020
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET
FIGURE 15 NUMBER OF NEW CASES IN 2018 IN FEMALES OF ALL AGES
FIGURE 16 AGEING EUROPE POPULATION (IN MILLION)
FIGURE 17 ESTIMATED LIFETIME CARE SPENDING
FIGURE 18 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 19 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 23 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 27 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 35 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 36 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 37 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 38 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: PRODUCT TYPE (2023-2030)
FIGURE 39 NORTH AMERICA MELANOMA CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












