North America Left Ventricular Assist Device (LVAD) Market Analysis and Size
A left ventricular assist device (LVAD) is a mechanical pump implanted in patients with heart failure. It helps the left bottom chamber of the heart (left ventricle) pump blood out of the ventricle to the aorta and the rest of the body. It is used for patients who have reached end-stage heart failure. The LVAD is surgically implanted, a battery-operated mechanical pump, which helps the left ventricle (main pumping chamber of the heart) pump blood to the rest of the body.
The occurrence of cardiovascular illnesses is growing at a rapid pace. It is estimated that advanced cardiovascular breakdown affects around 2.3% of North America's total population and is a severe socioeconomic burden. Further strong emphasis on R&D exercises aimed at the development of progressively enticing devices, as well as optimum government efforts, drive the left ventricular assist device (LVAD) market. Furthermore, the Canadian market is likely to benefit from low-intensity competition in the vendor landscape, making the country an attractive investment ground for firms looking to enter the sector of ventricular assist devices. The enormous pool of patients awaiting donor hearts for transplant gives commercial opportunities. However, there are dangers associated with the LVAD implant, and these hazards have an influence on market growth by acting as a restraining factor. Furthermore, the sector is currently confronted with a number of challenges, including barriers to DT adoption and safety concerns.
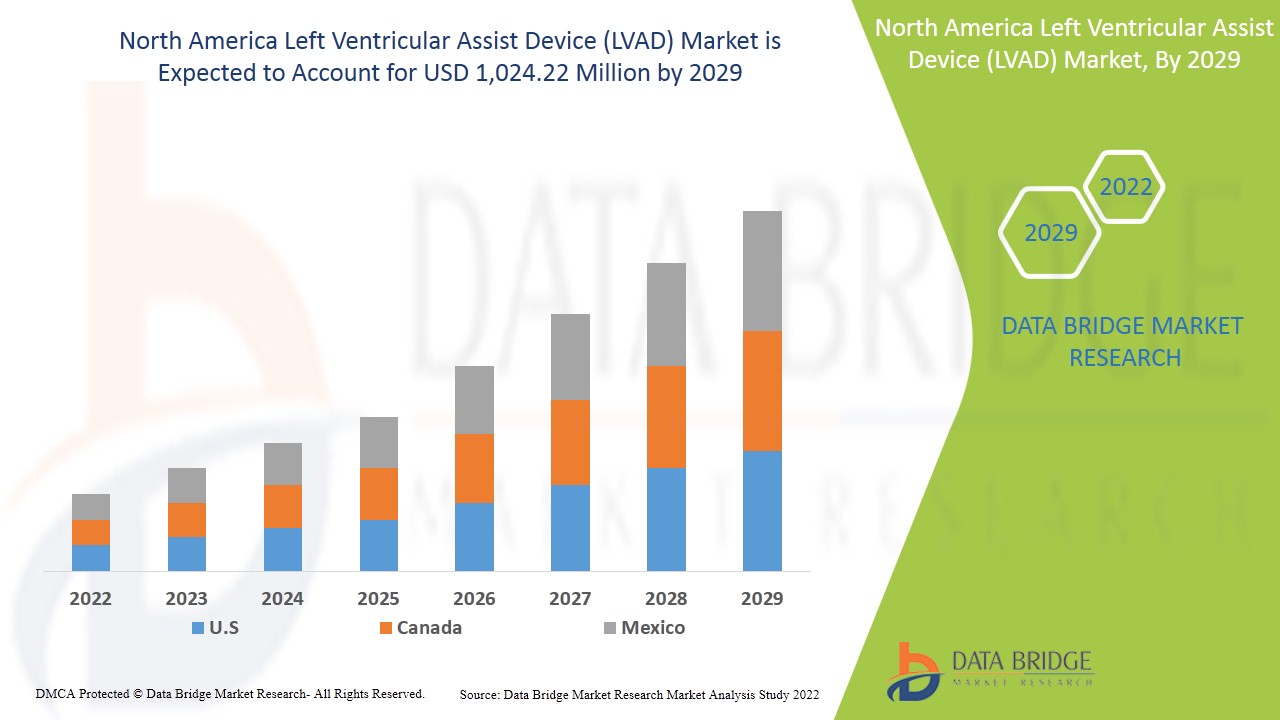
North America left ventricular assist device (LVAD) market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 9.5% in the forecast period of 2022 to 2029 and is expected to reach USD 1,024.22 million by 2029. Increasing technological advancements in the left ventricular assist device act as a driver for the left ventricular assist device (LVAD) market growth.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customisable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
By Product Type (Heart Pump, Controller, Batteries, and Wires), Therapy (Bridge-To-Transplant (BTT) Therapy, Destination Therapy, Bridge-To-Candidacy (BTC) Therapy, and Bridge-To-Recovery (BTR) Therapy), Age Group (Adult and Pediatric), Indication (Congestive Heart Failure, Congenital Heart Disease, Myocarditis, Cardiac Arrest, Familial Arrhythmias and Arrhythmic, Cardiomyopathies, Advanced Heart Failure, and Others), Generation (Second Generation Devices, Third Generation Devices, and First Generation Devices), Durability (Long-Term, Intermediate-Term, and Short-Term), Design (Axial and Centrifugal), Pulse Type (Nonpulsatile and Pulsatile), End User (Hospitals, Cardiac Cath Laboratories, Specialty Clinics, and Others), Distribution Channel (Direct Tender, Retail Sales, and Others) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
The major companies which are dealing in the market are ABIOMED, Abbott, Berlin Heart, Saft, Jarvik Heart, Inc., CorWave SA, and Evaheart, Inc., among others |
Market Definition
A left ventricular assist device (LVAD) is a mechanical pump implanted in patients with heart failure. It helps the left bottom chamber of the heart (left ventricle) pump blood out of the ventricle to the aorta and the rest of the body.
It is used for patients who have reached end-stage heart failure. The LVAD is surgically implanted, a battery-operated, mechanical pump, which helps the left ventricle (main pumping chamber of the heart) pump blood to the rest of the body. LVADs can be used as:
The high prevalence of cardiovascular diseases, increasing technological advancement, and rising healthcare expenditure are also propelling the growth of the North America left ventricular assist device (LVAD) market.
North America Left Ventricular Assist Device (LVAD) Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints and challenges. All of this is discussed in detail as below:
Drivers
- INCREASING GERIATRIC POPULATION, ALONG WITH THE RISING PREVALENCE OF CARDIAC DISEASES
The prevalence of cardiac diseases is also increasing with the growing geriatric population across North America. With an estimated 40 million blind individuals, most of them belong to the aged population. However, all the studies indicate a high prevalence of cardiac diseases in the geriatric population. With increasing age and the rising prevalence of cardiac disorders such as heart failure in the elderly population, proper treatment and medications are also rising. In addition, the growing population has increased the pressure on the healthcare system, due to which the need for care, services, and technologies is rising rapidly. Therefore, the increasing geriatric population is expected to drive North America left ventricular assist device (LVAD) market.
- PRESENCE OF FAVOURABLE REIMBURSEMENT POLICIES
The Centers for Medicare & Medicaid Services (CMS) has published a decision memorandum liberalizing criteria for coverage of ventricular assist devices (CMS, 2010). The CMS policy removes body size criteria and eases restrictions around the required duration of failed medical therapy and peak oxygen consumption. Henceforth, favorable reimbursement policies are expected to drive North America left ventricular assist device (LVAD) market in the forecast year.
Opportunities
- INCREASE IN HEALTHCARE EXPENDITURE
Healthcare expenditure has increased in North America as disposable income in the countries is increasing. Moreover, to accomplish the population requirements, the government bodies and healthcare organizations are taking initiatives by accelerating healthcare expenditure. The rise in healthcare expenditure simultaneously helps healthcare settings to improve their treatment facilities for various cardiovascular conditions and provide surgical treatment as the disorder has been highly prevalent in recent years.
The rise in healthcare expenditure has given the market an opportunity by providing more treatment options, free diagnostics, and different programs for promoting early diagnosis. Moreover, the healthcare expenditure by the government is focused on allocating resources towards strategies to improve management and patient outcomes to lower the growing economic burden.
Growing healthcare expenditure is also beneficial for further economic growth and healthcare sector growth, and it is primarily fruitful as it significantly affects the development of better and more advanced devices for such cardiology conditions. Thereby surge in healthcare expenditure is a greater opportunity for North America left ventricular assist device (LVAD) market.
Restraints/Challenges
- HIGH COST OF LVAD IMPLANTATION AND TREATMENT
LVADs in non-inotrope-dependent heart failure patients improve quality of life but substantially increase lifetime costs because of frequent readmissions and costly follow-up care. Left ventricular assist devices also increase employment and career, affecting finances.
The high cost of LVAD implantation and treatment poses a financial burden and challenges for a patient. Moreover, the high cost of follow-up care further increases the financial burden on the patient, which restrains the growth of the market.
This North America Left Ventricular Assist Device (LVAD) Market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market.
Recent Development
- On December 21, 2020, the U.S. Food and Drug Administration (FDA) approved updated labeling on December 17 for Abbott's HeartMate 3 left ventricular assist device (LVAD) to be used in pediatric patients with advanced refractory left ventricular heart failure
North America Left Ventricular Assist Device (LVAD) Market Scope
North America left ventricular assist device (LVAD) market is categorized into ten notable segments based on product type, therapy, age group, indication, generation, durability, design, pulse type, end-user, and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and insights to help them make strategic decisions for identifying core market applications.
BY PRODUCT TYPE
- HEART PUMP
- CONTROLLER
- BATTERIES
- WIRES
On the basis of product type, North America left ventricular assist device (LVAD) market is segmented into heart pump, controller, batteries, and wires. Batteries are further segmented into rechargeable and non-rechargeable.
BY THERAPY
- Bridge-To-Transplant (BTT) Therapy
- Destination Therapy
- Bridge-To-Candidacy (BTC) Therapy
- Bridge-To-Recovery (BTR) Therapy
On the basis of therapy, North America left ventricular assist device (LVAD) market is segmented bridge-to-transplant (BTT) therapy, destination therapy, bridge-to-candidacy (BTC) therapy, and bridge-to-recovery (BTR) therapy.
BY AGE GROUP
- Adult
- PAEDIATRIC
On the basis of age group, North America left ventricular assist device (LVAD) market is segmented into adult and paediatric.
BY INDICATION
- Congestive Heart Failure
- Congenital Heart Disease
- Myocarditis
- Cardiac Arrest
- Familial Arrhythymias and Arrhythmic
- Cardiomyopathies
- Advanced Heart Failure
- Others
On the basis of indication, North America left ventricular assist device (LVAD) market is segmented into congestive heart failure, congenital heart disease, myocarditis, cardiac arrest, familial arrhythymias and arrhythmic cardiomyopathies, advanced heart failure, and others.
BY GENERATION
- Second Generation Devices
- Third Generation Devices
- First Generation Devices
On the basis of generation, North America left ventricular assist device (LVAD) market is segmented into second-generation devices, third-generation devices, and first-generation devices.
BY DURABILITY
- Long-Term
- Intermediate-Term
- Short-Term
On the basis of durability, North America left ventricular assist device (LVAD) market is segmented into long-term, intermediate-term, and short-term.
BY DESIGN
- Axial
- Centrifugal
On the basis of design, North America left ventricular assist device (LVAD) market is segmented into axial and centrifugal.
BY PULSE TYPE
- Nonpulsatile
- Pulsatile
On the basis of pulse type, North America left ventricular assist device (LVAD) market is segmented into non-pulsatile and pulsatile.
BY END USER
- Hospitals
- Cardiac Cath Laboratories
- Specialty Clinics
- Others
On the basis of end user, North America left ventricular assist device (LVAD) market is segmented into hospitals, specialty clinics, cardiac cath laboratories, and others.
BY DISTRIBUTION CHANNEL
- DIRECT TENDER
- RETAIL SALES
- OTHERS
On the basis of distribution channel, North America left ventricular assist device (LVAD) market is segmented into direct tender, retail sales, and others.
North America Left Ventricular Assist Device (LVAD) Market Regional Analysis/Insights
North America left ventricular assist device (LVAD) market is analyzed and market size insights and trends are provided by country, product type, therapy, age group, indication, generation, durability, design, pulse type, end-user, and distribution channel as referenced above.
North America left ventricular assist device (LVAD) market, it includes countries such as U.S., Canada and Mexico and is expected to grow due to an increase in cases of cardiac disorders and the growing geriatric population. The increasing risk of ventricular disorders are also driving the market growth.
The US is expected to dominate North America left ventricular assist device (LVAD) market due to the presence of advanced technology, high prevalence of cardiac disorders and established health care system.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of other brands and their challenges faced due to large or scarce competition from local and domestic brands the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Left Ventricular Assist Device (LVAD) Market Share Analysis
North America Left Ventricular Assist Device (LVAD) Market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, country presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus related to the osteoporosis drugs market.
Some of the major players operating in North America left ventricular assist device (LVAD) market are ABIOMED, Abbott, Berlin Heart, Saft, Jarvik Heart, Inc., CorWave SA, and Evaheart, Inc., among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S
4.3 INDUSTRIAL INSIGHTS:
5 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: REGULATIONS
5.1 REGULATION IN U.S.
5.1.1 GUIDELINES-
5.2 REGULATION IN CANADA
5.2.1 GUIDELINES FOR THE MANUFACTURERS:
5.3 REGULATION IN MEXICO
5.3.1 GUIDELINES FOR THE MANUFACTURERS:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING GERIATRIC POPULATION ALONG WITH RISING PREVALENCE OF CARDIAC DISEASES
6.1.2 PRESENCE OF FAVOURABLE REIMBURSEMENT POLICIES
6.1.3 CHANGING LIFESTYLE TRIGGERS THE DEVELOPMENT OF CARDIOVASCULAR DISEASES
6.2 RESTRAINTS
6.2.1 HIGH COST OF LVAD IMPLANTATION AND TREATMENT
6.2.2 COMPLICATIONS AND RISKS ASSOCIATED WITH LVAD
6.3 OPPORTUNITIES
6.3.1 INCREASE IN HEALTHCARE EXPENDITURE
6.3.2 INCREASE IN MINIMALLY INVASIVE PROCEDURE
6.3.3 INCREASING SHORTAGE OF ORGAN DONORS
6.3.4 TECHNOLOGICAL ADVANCEMENTS IN LEFT VENTRICULAR ASSIST DEVICES
6.4 CHALLENGES
6.4.1 ONGOING COVID-19
6.4.2 INCREASE IN PRODUCT RECALL
7 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 HEART PUMP
7.3 CONTROLLER
7.4 BATTERIES
7.4.1 RECHARGEABLE
7.4.2 NON-RECHARGEABLE
7.5 WIRES
8 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY
8.1 OVERVIEW
8.2 BRIDGE-TO-TRANSPLANT (BTT) THERAPY
8.2.1 HEART PUMP
8.2.2 CONTROLLER
8.2.3 BATTERIES
8.2.4 WIRES
8.3 DESTINATION THERAPY
8.3.1 HEART PUMP
8.3.2 CONTROLLER
8.3.3 BATTERIES
8.3.4 WIRES
8.4 BRIDGE-TO-CANDIDACY (BTC) THERAPY
8.4.1 HEART PUMP
8.4.2 CONTROLLER
8.4.3 BATTERIES
8.4.4 WIRES
8.5 BRIDGE-TO-RECOVERY (BTR) THERAPY
8.5.1 HEART PUMP
8.5.2 CONTROLLER
8.5.3 BATTERIES
8.5.4 WIRES
9 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP
9.1 OVERVIEW
9.2 ADULT
9.2.1 19-39 YEARS
9.2.2 40-59 YEARS
9.2.3 60-79 YEARS
9.2.4 ABOVE 80 YEARS
9.3 PEDIATRIC
10 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION
10.1 OVERVIEW
10.2 SECOND GENERATION DEVICES
10.3 THIRD GENERATION DEVICES
10.4 FIRST GENERATION DEVICES
11 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN
11.1 OVERVIEW
11.2 AXIAL
11.3 CENTRIFUGAL
12 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION
12.1 OVERVIEW
12.2 CONGESTIVE HEART FAILURE
12.2.1 HEART PUMP
12.2.2 CONTROLLER
12.2.3 BATTERIES
12.2.4 WIRES
12.3 CONGENITAL HEART DISEASE
12.3.1 HEART PUMP
12.3.2 CONTROLLER
12.3.3 BATTERIES
12.3.4 WIRES
12.4 MYOCARDITIS
12.4.1 HEART PUMP
12.4.2 CONTROLLER
12.4.3 BATTERIES
12.4.4 WIRES
12.5 CARDIAC ARREST
12.5.1 HEART PUMP
12.5.2 CONTROLLER
12.5.3 BATTERIES
12.5.4 WIRES
12.6 FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES
12.6.1 HEART PUMP
12.6.2 CONTROLLER
12.6.3 BATTERIES
12.6.4 WIRES
12.7 ADVANCED HEART FAILURE
12.7.1 HEART PUMP
12.7.2 CONTROLLER
12.7.3 BATTERIES
12.7.4 WIRES
12.8 OTHERS
13 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DURABILITY
13.1 OVERVIEW
13.2 LONG-TERM
13.3 INTERMEDIATE-TERM
13.4 SHORT-TERM
14 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY PULSE TYPE
14.1 OVERVIEW
14.2 NONPULSATILE
14.3 PULSATILE
15 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITAL
15.3 CARDIAC CATH LABORATORIES
15.4 SPECIALTY CLINICS
15.5 OTHERS
16 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY NORTH AMERICA
17.1 NORTH AMERICA
17.1.1 U.S.
17.1.2 CANADA
17.1.3 MEXICO
18 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
19 SWOT ANALYSIS
20 COMPANY PROFILE
20.1 ABIOMED
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.1.5.1 FDA APPROVAL
20.1.5.2 FDA APPROVAL
20.2 ABBOTT
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.2.5.1 SUPPLY INCREMENT
20.2.5.2 BREAK THROUGH DEVICE DESIGNATION
20.3 BERLIN HEART
20.3.1 COMPANY SNAPSHOT
20.3.2 COMPANY SHARE ANALYSIS
20.3.3 PRODUCT PORTFOLIO
20.3.4 RECENT DEVELOPMENT
20.3.4.1 PRODUCT APPROVAL
20.4 SAFT (A SUBSIDIARY OF TOTALENERGIES)
20.4.1 COMPANY SNAPSHOT
20.4.2 REVENUE ANALYSIS
20.4.3 COMPANY SHARE ANALYSIS
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENT
20.4.5.1 PARTNERSHIP
20.5 JARVIK HEART, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 COMPANY SHARE ANALYSIS
20.5.3 PRODUCT PORTFOLIO
20.5.4 RECENT DEVELOPMENT
20.5.4.1 FDA APPROVAL
20.6 CORWAVE SA
20.6.1 COMPANY SNAPSHOT
20.6.2 PRODUCT PORTFOLIO
20.6.3 RECENT DEVELOPMENT
20.6.3.1 EXPANSION
20.7 EVAHEART, INC
20.7.1 COMPANY SNAPSHOT
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.7.3.1 PRODUCT TRIAL
21 QUESTIONNAIRE
22 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 NORTH AMERICA BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 24 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 25 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 26 U.S. BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 27 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 28 U.S. BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 29 U.S. DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 30 U.S. BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 31 U.S. BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 32 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 33 U.S. ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 34 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 35 U.S. CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 36 U.S. CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 37 U.S. MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 38 U.S. CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 39 U.S. FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 40 U.S. ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 41 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 42 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 43 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 44 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 45 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 46 U.S. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 47 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 48 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 49 CANADA BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 50 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 51 CANADA BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 52 CANADA DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 53 CANADA BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 54 CANADA BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 55 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 56 CANADA ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 57 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 58 CANADA CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 59 CANADA CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 60 CANADA MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 61 CANADA CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 62 CANADA FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 63 CANADA ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 64 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 65 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 66 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 67 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 68 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 69 CANADA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 70 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 71 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 72 MEXICO BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 73 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 74 MEXICO BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 75 MEXICO DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 76 MEXICO BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 77 MEXICO BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 78 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 79 MEXICO ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 80 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 81 MEXICO CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 82 MEXICO CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 83 MEXICO MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 84 MEXICO CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 85 MEXICO FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 86 MEXICO ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 87 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 88 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 89 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 90 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 91 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 92 MEXICO LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: NORTH AMERICA VS REGIONAL ANALYSIS
FIGURE 5 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET:VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE OF CARDIOVASCULAR DISEASE AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 HEART PUMP SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
FIGURE 14 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2021
FIGURE 15 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2020-2029 (USD MILLION)
FIGURE 16 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 17 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2021
FIGURE 19 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2020-2029 (USD MILLION)
FIGURE 20 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, CAGR (2022-2029)
FIGURE 21 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 22 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2021
FIGURE 23 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2020-2029 (USD MILLION)
FIGURE 24 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, CAGR (2022-2029)
FIGURE 25 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 26 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2021
FIGURE 27 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2020-2029 (USD MILLION)
FIGURE 28 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, CAGR (2022-2029)
FIGURE 29 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, LIFELINE CURVE
FIGURE 30 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2021
FIGURE 31 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2020-2029 (USD MILLION)
FIGURE 32 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, CAGR (2022-2029)
FIGURE 33 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, LIFELINE CURVE
FIGURE 34 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2021
FIGURE 35 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2020-2029 (USD MILLION)
FIGURE 36 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, CAGR (2022-2029)
FIGURE 37 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 38 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2021
FIGURE 39 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2020-2029 (USD MILLION)
FIGURE 40 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, CAGR (2022-2029)
FIGURE 41 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, LIFELINE CURVE
FIGURE 42 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2021
FIGURE 43 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2020-2029 (USD MILLION)
FIGURE 44 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, CAGR (2022-2029)
FIGURE 45 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, LIFELINE CURVE
FIGURE 46 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2021
FIGURE 47 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 48 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, CAGR (2022-2029)
FIGURE 49 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, LIFELINE CURVE
FIGURE 50 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 51 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 52 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 53 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 54 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SNAPSHOT (2021)
FIGURE 55 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021)
FIGURE 56 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2022 & 2029)
FIGURE 57 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021 & 2029)
FIGURE 58 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 59 NORTH AMERICA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.