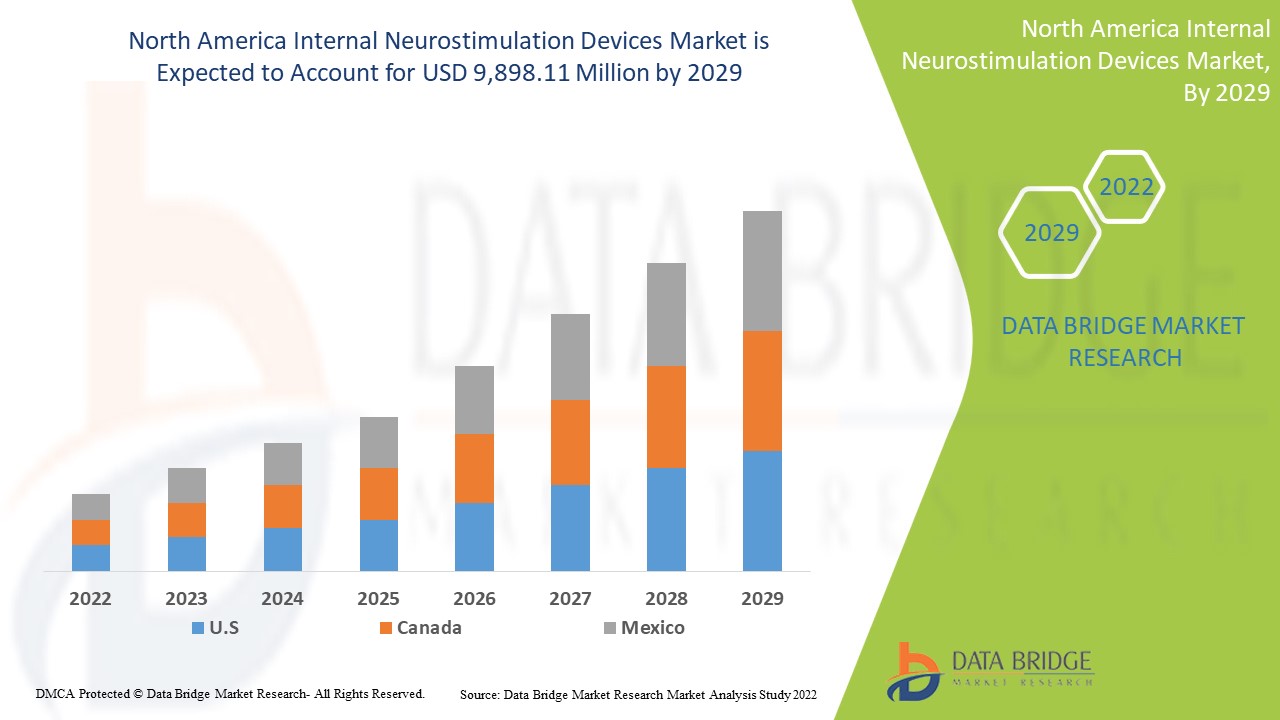
North America Internal Neurostimulation Devices Market Analysis and Insights
The rise in demand for internal neurostimulation devices as add-on therapy, increase in prevalence and incidence of neurological diseases, increased funding for the neurostimulation devices, technological advancements in the internal neurostimulation devices, and rise in product approvals are expected to drive market growth.
The strategic initiatives by market players and increased funding by public and private market players for internal neurostimulation devices are expected to create opportunities for market growth. However, the lack of skilled and trained experts in internal neurostimulation devices is expected to restrain the segment's growth. The availability of alternate imaging devices is expected to challenge the market growth.
Data Bridge Market Research analyzes that the North America internal neurostimulation devices market will grow at a CAGR of 20.5% and USD 9,898.11 Million during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2024) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
By Product Type (Spinal Cord Stimulation (SCS), Deep Brain Stimulation, Vagus Nerve Stimulation, Sacral Nerve Stimulation and Gastric Electric Stimulation), Distribution Channel (Direct Tender and Third Party Service Provider) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
Medtronic, LivaNova PLC, Abbott, ONWARD, Sequana Medical NV, CIRTEC, Valencia Technologies, Nalu Medical, Inc., NEVRO CORP., Stimwave LLC, MicroTransponder Inc., Newronika S.p.A., Microsemi (a subsidiary of Microchip Technology Inc.), Boston Scientific Corporation, Inspire Medical Systems, Inc, Integer Holdings Corporation, BlueWind Medical, Micro-Leads, Axonics, Inc., among others |
Market Definition
An internal neurostimulation device is a surgically placed device. It delivers mild electrical signals to the epidural space near your spine through one or more thin wires called leads. Neurostimulation provides pain relief by disrupting the pain signals traveling between the spinal cord and the brain.
Neurostimulation devices include invasive and noninvasive approaches involving electrical stimulation to drive neural function within a circuit. The increased demand for internal neurostimulation devices is due to next-generation technological progressions in the neurostimulation devices, providing much-needed therapeutic relief to an unprecedented number of people affected by debilitating neurologic and psychiatric disorders worldwide. The rise of modern-day neuromodulation therapies has increased over half a century, rich with serendipitous discoveries and technological advances that have led to different neurostimulation strategies. Within the past two decades, innovation in medical device technology has begun to drive the evolution of these neurostimulation systems at a more accelerated pace.
The convenient internal neurostimulation device used by the patients is vagus nerve stimulation. The vagus neurostimulation device uses a device to stimulate the vagus nerve with electrical impulses. An implantable vagus nerve stimulator is currently FDA-approved to treat epilepsy and depression. There's one vagus nerve on each side of the body, running from the brainstem through the neck to the chest and abdomen. In the future, software advancements such as closed-loop stimulation and remote programming will enable internal neurostimulation devices to be a more personalized and accessible technology. The future of internal neurostimulation devices is expected to improve quality of life further.
North America Internal Neurostimulation Devices Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Increased Awareness About Treatment of Chronic Urinary Incontinence
Increasing awareness among patients and health care providers and greater access to continence services were key factors in improving care delivery. Urinary incontinence (UI) and lower urinary tract symptoms (LUTS) are common and cause distress for many women and men of all ages. The increased awareness of implant internal neurostimulation devices in neurology signifies the increase in research and development-related investments for the discovery and development of advanced, pain-free internal neurostimulation devices, which is expected to boost market growth.
- Technological Advancements in the Internal Neurostimulation Devices
The technological developments in internal neurostimulation devices utilize neuromodulation technology, which directly delivers the electrical or pharmaceutical agents to a target area. The neuromodulation and internal neurostimulation devices and treatments are life-changing. Technological developments modulate neural functions in a programmable manner and regulate disordered neural activity. The results can be delivered with minimum error.
For instance,
- In May 2022, ONWARD introduced the ARC implantable pulse generator (IPG) to stimulate the spinal cord to restore movement and autonomic function for people with SCI and other conditions that impact mobility
The approval for the internal neurostimulation devices would result in the device being declared safe to use and ready for post-marketing approval. It would result in the supply and distribution of MRI machines to the developing markets in the U.S. demographics. Hence, the rise in product approvals is expected to propel the North America internal neurostimulation devices market growth.
Opportunities
- Recent Product Developments in the Internal Neurostimulation Devices
The growth curve for the North America internal neurostimulation devices market is following an upward trend due to the demand for effective neurovascular therapies is steadily increasing due to the increasing prevalence of neurovascular diseases (such as epilepsy, brain stroke, and cerebral aneurysm) and the severity of diseases (such as discharge and surrounding disorders) in target patients. Due to the rising prevalence and increasing awareness about the seriousness of these diseases, various equipment or devices are manufactured or are under clinical trials.
Thus, product development in recent years has shown the potential of these technologies, and the companies working in this market are trying to get more advanced products, which will act as an opportunity for market growth.
- Strategic Initiatives by the Key Market Player
The demand for internal neurostimulation devices is increasing in the market owing to the increased levels of research and development along with the growth of the North America internal neurostimulation devices market aided by the desire for innovative medications. Thus, the top market players have implemented a new strategy by developing new devices and equipment, collaborating with other players in the market, and improving business operations and profitability.
- In January 2021, Boston Scientific began shipping their Wave Writer Alpha spinal line trigger frameworks to the U.S.
Thus, the companies operating globally in the neurostimulation devices market are adopting collaboration to increase their product portfolio with advanced technology-rich products to boost their business in various dimensions. Thus, strategic initiatives by key market players are expected to offer significant opportunities for the market players operating in the North America internal neurostimulation devices market.
Restraints/Challenges
- Risks Associated with the Implantation of these Devices
Medical implants carry several hazards, including those related to surgery during installation or removal, infection, and implant failure. The materials used in implants can potentially cause responses in some persons. Every surgical procedure carries some risk. These include bruising, discomfort, swelling, and redness at the surgery site. Thereby will proportionally hamper the growth of implant devices. The increasing awareness about the rising risks related to neurostimulation implants, therefore, may hamper the market's growth.
For instance,
- Implant failure
- Surgical risks during placement or removal
- Risk of implantable device hijacking
- Rising infections
- Materials used in implants might show adverse reactions in patients
The risks mentioned above may hamper the growth of the internal neurostimulation devices market as the risks concerned with the patients are of keen importance. Thus, the awareness of the potential risks of implantable devices is a challenge for the North America internal neurostimulation devices market.
- Lack of Skilled Healthcare Professionals
Neurostimulation devices are implantable, programmable medical devices that deliver electrical stimulation to specific parts of the patient's brain, spinal cord, or peripheral nervous system to help treat various conditions, including chronic pain, movement disorders, epilepsy, and Parkinson's disease. These powerful technologies require a high-cost developmental procedure and skilled professionals to handle sensitive devices. The replacement should be done every 3-6 years after implantation, which is a cost burden and is typically out of pocket for most patients.
Only a relatively small number of patients in poorer nations can afford neurological therapy due to high prices, a weak reimbursement environment, and a lack of skilled healthcare resources. As a result, healthcare facilities are hesitant to spend money on novel or cutting-edge technology, limiting the expansion of the market. Thus, these challenges may hamper the growth of the market.
COVID-19 Impact on the North America Internal Neurostimulation Devices Market
During the pandemic, the North America Internal neurostimulation devices sector focuses on using a combination of biology and information technology. During the COVID-19 pandemic, new therapeutic challenges have been added to the usual ones in the internal neurostimulation devices. Patients with an implantable device for intrathecal infusion need a refill of the pump to avoid abstinence syndrome. Patients with neurostimulation implants can need checkups in case of infection, wound dehiscence, or lead migration.
Recent Developments
- In January 2022, Medtronic received product approval from the Food and Drug Administration (FDA) for Intellis rechargeable neurostimulator, a spinal cord stimulation therapy for treating chronic pain resulting from diabetic peripheral neuropathy. The product approval received resulted in the addition of a new product in the spinal cord stimulation product category. The approval is expected for post-market approval in the U.S. market
- In July 2022, Abbott received approval from the Food and Drug Administration (FDA) for the Infinity deep brain stimulation system to treat depression. The approval received resulted in the rise in the initiation of pre and post-marketing approval and the addition of a new product to the product portfolio
North America Internal Neurostimulation Devices Market Scope
North America internal neurostimulation devices market is categorized into product type and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Spinal Cord Stimulation (SCS)
- Deep Brain Stimulation
- Vagus Nerve Stimulation
- Sacral Nerve Stimulation
- Gastric Electric Stimulation
On the basis of product type, the North America internal neurostimulation devices market is segmented into spinal cord stimulation (SCS), deep brain stimulation, vagus nerve stimulation, sacral nerve stimulation, gastric electric stimulation.
Distribution Channel
- Direct Tender
- Third Party Service Provider
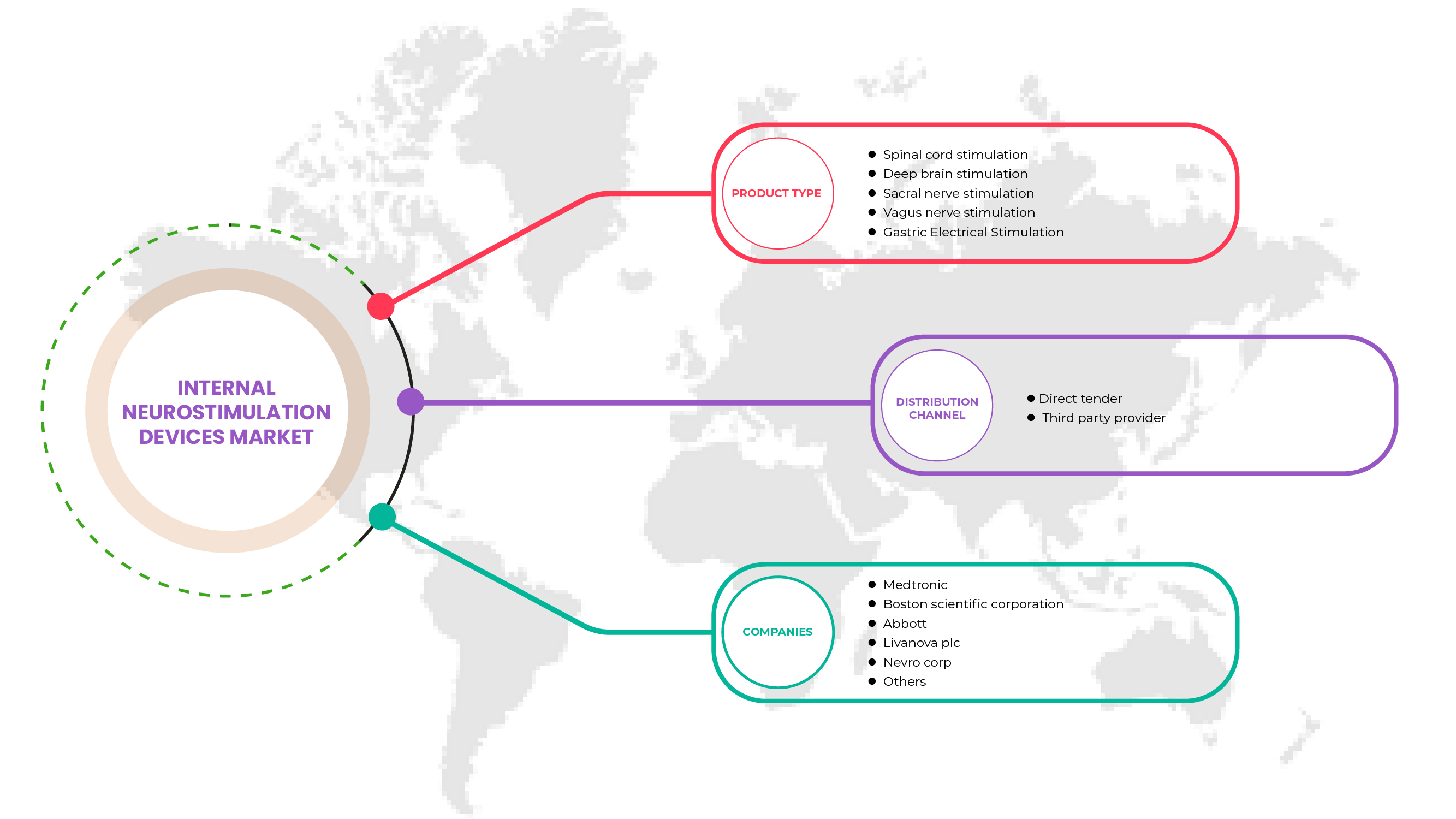
On the basis of distribution channel, the North America internal neurostimulation devices market is segmented into direct tender and third party service provider.
North America Internal Neurostimulation Devices Market Regional Analysis/Insights
North America internal neurostimulation devices market is analyzed, and regions provide market size insights and trends by country, product type, and distribution channel, as referenced above.
Some countries covered in the North America Internal neurostimulation devices market are the U.S., Canada, and Mexico. U.S. is expected to dominate the market due to a rise in research and development activity, integrated internal neurostimulation device requirements in neurology and urinary incontinence treatment, and a rise in public-private sector funding for neurostimulation devices.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North American brands and their challenges faced due to large or scarce competition from local and domestic brands impact sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Internal Neurostimulation Devices Market Share Analysis
North America internal neurostimulation devices market competitive landscape provides details on the competitor. Details include company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, the North American presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus related to the North America internal neurostimulation devices market.
Some of the major players operating in the North America internal neurostimulation devices market are Medtronic, LivaNova PLC, Abbott, ONWARD, Sequana Medical NV, CIRTEC, Valencia Technologies, Nalu Medical, Inc., NEVRO CORP., Stimwave LLC, MicroTransponder Inc., Newronika S.p.A., Microsemi (a subsidiary of Microchip Technology Inc.), Boston Scientific Corporation, Inspire Medical Systems, Inc, Integer Holdings Corporation, BlueWind Medical, Micro-Leads, Axonics, Inc., among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHIC SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 RESEARCH METHODOLOGY
2.6 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.7 DBMR MARKET POSITION GRID
2.8 THE CATEGORY VS TIME GRID
2.9 SECONDARY SOURCES
2.1 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 PIPELINE ANALYSIS FOR INTERNAL NEUROSTIMULATION DEVICES MARKET
5 REGULATIONS OF NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET
6 EPIDEMIOLOGY OF DISEASES
6.1 INCIDENCE OF ISCHEMIA
6.2 INCIDENCE OF PARKINSON'S DISEASE
6.3 INCIDENCE OF FAILED BACK SYNDROME
6.4 INCIDENCE OF TREMOR
6.5 INCIDENCE OF DEPRESSION
6.6 INCIDENCE OF URINE INCONTINENCE
6.7 INCIDENCE OF FECAL INCONTINENCE
6.8 INCIDENCE RATE OF EPILEPSY
6.9 INCIDENCE OF GASTROPARESIS
6.1 PREVALENCE OF OBESITY
7 EPIDEMIOLOGY OF NEUROSTIMULATION PROCEDURES
7.1 NUMBER OF SPINAL CORD STIMULATION (SCS) PROCEDURES
7.1.1 NUMBER OF TEST PROCEDURES
7.1.2 NUMBER OF IMPLANTATION PROCEDURES
7.2 NUMBER OF DEEP BRAIN STIMULATION PROCEDURES
7.3 NUMBER OF VAGUS NERVE STIMULATION PROCEDURES
7.4 NUMBER OF SACRAL NEVER STIMULATION PROCEDURES
7.5 NUMBER OF TRANSCRANIAL MAGNETIC STIMULATION (TMS) PROCEDURES
7.6 NUMBER OF INTERMITTENT THETA BURST STIMULATION (ITBS) PROCEDURES
7.7 NUMBER OF TRANSCRANIAL DIRECT ELECTRICAL STIMULATION (TDCS) PROCEDURES
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISE IN PREVALENCE AND INCIDENCE OF NEUROLOGICAL DISORDERS
8.1.2 DEMAND FOR INTERNAL NEUROSTIMULATION DEVICES AS A ADD ON THERAPY
8.1.3 INCREASED FUNDING FOR THE NEUROSTIMULATION DEVICES
8.1.4 TECHNOLOGICAL ADVANCEMENTS IN THE INTERNAL NEUROSTIMULATION DEVICES
8.1.5 RISE IN PRODUCT APPROVALS
8.2 RESTRAINTS
8.2.1 RISE IN COST OF THE DEEP BRAIN STIMULATION DEVICES
8.2.2 RISKS NOTICED WHILE USING THE INTERNAL NEUROSTIMULATION DEVICES
8.2.3 RISE IN PRODUCT RECALL
8.2.4 AVAILABILITY OF ALTERNATE IMAGING DIAGNOSTIC DEVICES
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYER
8.3.2 RECENT PRODUCT DEVELOPMENTS IN THE INTERNAL NEUROSTIMULATION DEVICES
8.3.3 DEMAND FOR MINIMALLY INVASIVE SURGERY
8.4 CHALLENGES
8.4.1 RISKS ASSOCIATED WITH THE IMPLANTATION OF THESE DEVICES
8.4.2 LACK OF SKILLED HEALTHCARE PROFESSIONALS
9 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 SPINAL CORD STIMULATION
9.2.1 SPINAL CORD STIMULATION, BY TYPE
9.2.1.1 BATTERY
9.2.1.1.1 RECHARGEABLE
9.2.1.1.2 NON-RECHARGEABLE
9.2.1.2 LEAD
9.2.1.2.1 PERCUTANEOUS
9.2.1.2.2 PADDLE
9.2.2 SPINAL CORD STIMULATION, BY APPLICATION
9.2.2.1 ISCHEMIA
9.2.2.2 CHRONIC LOW BACK PAIN (CLBP)
9.2.2.3 DIABETIC NEUROPATHY
9.2.2.4 FAILED BACK SYNDROME
9.3 DEEP BRAIN STIMULATION
9.3.1 DEEP BRAIN STIMULATION, BY TYPE
9.3.1.1 SINGLE CHANNEL DEEP BRAIN STIMULATOR
9.3.1.2 DOUBLE CHANNEL DEEP BRAIN STIMULATOR
9.3.1.3 BATTERY
9.3.1.3.1 RECHARGEABLE
9.3.1.3.2 NON-RECHARGEABLE
9.3.1.4 LEAD
9.3.2 DEEP BRAIN STIMULATION, BY APPLICATION
9.3.2.1 PARKINSON’S DISEASE
9.3.2.2 TREMOR
9.3.2.3 DEPRESSION
9.4 SACRAL NERVE STIMULATION
9.4.1 SACRAL NERVE STIMULATION, BY TYPE
9.4.1.1 BATTERY
9.4.1.2 LEAD
9.5 SACRAL NERVE STIMULATION, BY APPLICATION
9.5.1 URINE INCONTINENCE
9.5.2 FECAL INCONTINENCE
9.6 VAGUS NERVE STIMULATION
9.6.1 VAGUS NERVE STIMULATION, BY TYPE
9.6.1.1 BATTERY
9.6.1.2 LEAD
9.6.2 VAGUS NERVE STIMULATION, BY APPLICATION
9.6.2.1 EPILEPSY
9.6.2.2 OTHERS
9.7 GASTRIC ELECTRICAL STIMULATION
9.7.1 GASTRIC ELECTRICAL STIMULATION, BY TYPE
9.7.1.1 BATTERY
9.7.1.2 LEAD
9.7.2 GASTRIC ELECTRICAL STIMULATION, BY APPLICATION
9.7.2.1 GASTROPARESIS
9.7.2.2 OTHERS
10 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 THIRD PARTY PROVIDER
11 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION
11.1 NORTH AMERICA
11.1.1 U.S.
11.1.2 CANADA
11.1.3 MEXICO
12 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 MEDTRONIC (2021)
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENTS
14.2 BOSTON SCIENTIFIC CORPORATION (2021)
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENTS
14.3 ABBOTT (2021)
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 PRODUCT PORTFOLIO
14.3.5 RECENT DEVELOPMENTS
14.4 LIVANOVA PLC (2021)
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENTS
14.5 NEVRO CORP. (2021)
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENTS
14.6 AXONICS, INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENTS
14.7 ALEVA NEUROTHERAPEUTICS
14.7.1 COMPANY SNAPSHOT
14.7.2 PRODUCT PORTFOLIO
14.7.3 RECENT DEVELOPMENTS
14.8 BIONIC VISION TECHNOLOGIES.
14.8.1 COMPANY SNAPSHOT
14.8.2 PRODUCT PORTFOLIO
14.8.3 RECENT DEVELOPMENTS
14.9 BLUEWIND MEDICAL
14.9.1 COMPANY SNAPSHOT
14.9.2 PRODUCT PORTFOLIO
14.9.3 RECENT DEVELOPMENTS
14.1 BIOINDUCTION
14.10.1 COMPANY SNAPSHOT
14.10.2 PRODUCT PORTFOLIO
14.10.3 RECENT DEVELOPMENT
14.11 CIRTEC
14.11.1 COMPANY SNAPSHOT
14.11.2 PRODUCT PORTFOLIO
14.11.3 RECENT DEVELOPMENTS
14.12 FINETECH MEDICAL
14.12.1 COMPANY SNAPSHOT
14.12.2 PRODUCT PORTFOLIO
14.12.3 RECENT DEVELOPMENTS
14.13 GIMER MEDICAL
14.13.1 COMPANY SNAPSHOT
14.13.2 PRODUCT PORTFOLIO
14.13.3 RECENT DEVELOPMENTS
14.14 INSPIRE MEDICAL SYSTEMS, INC.
14.14.1 COMPANY SNAPSHOT
14.14.2 REVENUE ANALYSIS
14.14.3 PRODUCT PORTFOLIO
14.14.4 RECENT DEVELOPMENTS
14.15 INTEGER HOLDINGS CORPORATION
14.15.1 COMPANY SNAPSHOT
14.15.2 REVENUE ANALYSIS
14.15.3 PRODUCT PORTFOLIO
14.15.4 RECENT DEVELOPMENT
14.16 INBRAIN NEUROELECTRONICS
14.16.1 COMPANY SNAPSHOT
14.16.2 PRODUCT PORTFOLIO
14.16.3 RECENT DEVELOPMENT
14.17 MAINSTAY MEDICAL
14.17.1 COMPANY SNAPSHOT
14.17.2 REVENUE ANALYSIS
14.17.3 PRODUCT PORTFOLIO
14.17.4 RECENT DEVELOPMENTS
14.18 MICROSEMI (A WHOLLY OWNDED SUBSIDIARY OF MICROCHIP TECHNOLOGY INC.)
14.18.1 COMPANY SNAPSHOT
14.18.2 REVENUE ANALYSIS
14.18.3 PRODUCT PORTFOLIO
14.18.4 RECENT DEVELOPMENTS
14.19 MICROTRANSPONDER INC
14.19.1 COMPANY SNAPSHOT
14.19.2 PRODUCT PORTFOLIO
14.19.3 RECENT DEVELOPMENT
14.2 MICRO-LEADS
14.20.1 COMPANY SNAPSHOT
14.20.2 PRODUCT PORTFOLIO
14.20.3 RECENT DEVELOPMENT
14.21 NALU MEDICAL, INC
14.21.1 COMPANY SNAPSHOT
14.21.2 PRODUCT PORTFOLIO
14.21.3 RECENT DEVELOPMENT
14.22 NEURONANO AB
14.22.1 COMPANY SNAPSHOT
14.22.2 PRODUCT PORTFOLIO
14.22.3 RECENT DEVELOPMENTS
14.23 NEURIMPULSE S.R.L.
14.23.1 COMPANY SNAPSHOT
14.23.2 PRODUCT PORTFOLIO
14.23.3 RECENT DEVELOPMENTS
14.24 NEWRONIKA S.P.A.
14.24.1 COMPANY SNAPSHOT
14.24.2 PRODUCT PORTFOLIO
14.24.3 RECENT DEVELOPMENTS
14.25 OPTOGENTECH GMBH.
14.25.1 COMPANY SNAPSHOT
14.25.2 PRODUCT PORTFOLIO
14.25.3 RECENT DEVELOPMENTS
14.26 ONWARD
14.26.1 COMPANY SNAPSHOT
14.26.2 PRODUCT PORTFOLIO
14.26.3 RECENT DEVELOPMENT
14.27 STIMWAVE LLC (2021)
14.27.1 COMPANY SNAPSHOT
14.27.2 PRODUCT PORTFOLIO
14.27.3 RECENT DEVELOPMENTS
14.28 SEQUANA MEDICAL NV (2021)
14.28.1 COMPANY SNAPSHOT
14.28.2 REVENUE ANALYSIS
14.28.3 PRODUCT PORTFOLIO
14.28.4 RECENT DEVELOPMENTS
14.29 VALENCIA TECHNOLOGIES
14.29.1 COMPANY SNAPSHOT
14.29.2 PRODUCT PORTFOLIO
14.29.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA PREVALENCE OF OBESITY
TABLE 2 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 7 NORTH AMERICA BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 8 NORTH AMERICA LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 10 NORTH AMERICA LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 11 NORTH AMERICA SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 15 NORTH AMERICA DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 16 NORTH AMERICA BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 18 NORTH AMERICA BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 19 NORTH AMERICA DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 23 NORTH AMERICA SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 24 NORTH AMERICA SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 28 NORTH AMERICA VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 29 NORTH AMERICA VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 33 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 34 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA DIRECT TENDER IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA THIRD PARTY PROVIDER IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 43 NORTH AMERICA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 44 NORTH AMERICA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 46 NORTH AMERICA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 47 NORTH AMERICA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 50 NORTH AMERICA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 51 NORTH AMERICA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 53 NORTH AMERICA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 54 NORTH AMERICA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 NORTH AMERICA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 57 NORTH AMERICA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 58 NORTH AMERICA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 59 NORTH AMERICA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 NORTH AMERICA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 61 NORTH AMERICA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 62 NORTH AMERICA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 63 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 65 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 66 NORTH AMERICA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 NORTH AMERICA NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 68 U.S. NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 69 U.S. SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 70 U.S. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 71 U.S. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 72 U.S. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 73 U.S. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 74 U.S. LEAD IN N NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 75 U.S. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 76 U.S. SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 77 U.S. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 78 U.S. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 79 U.S. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 80 U.S. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 81 U.S. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 82 U.S. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 83 U.S. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 84 U.S. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 85 U.S. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 86 U.S. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 87 U.S. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 88 U.S. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 89 U.S. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 90 U.S. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 91 U.S. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 92 U.S. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 93 U.S. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 94 U.S. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 95 U.S. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 96 U.S. NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 97 CANADA NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 98 CANADA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 99 CANADA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 100 CANADA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 101 CANADA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 102 CANADA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 103 CANADA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 104 CANADA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 105 CANADA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 106 CANADA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 107 CANADA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 108 CANADA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 109 CANADA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 110 CANADA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 111 CANADA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 112 CANADA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 113 CANADA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 114 CANADA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 115 CANADA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 116 CANADA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 117 CANADA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 118 CANADA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 119 CANADA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 120 CANADA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 121 CANADA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 122 CANADA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 123 CANADA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 124 CANADA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 125 CANADA NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 126 MEXICO NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 127 MEXICO SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 128 MEXICO BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 129 MEXICO BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 130 MEXICO BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 131 MEXICO LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 132 MEXICO LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 133 MEXICO LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 134 MEXICO SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 135 MEXICO DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 136 MEXICO DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 137 MEXICO DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 138 MEXICO BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 139 MEXICO BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 140 MEXICO BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 141 MEXICO DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 142 MEXICO SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 143 MEXICO SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 144 MEXICO SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 145 MEXICO SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 146 MEXICO VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 147 MEXICO VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 148 MEXICO VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 149 MEXICO VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 150 MEXICO GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 151 MEXICO GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 152 MEXICO GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 153 MEXICO GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 154 MEXICO NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: GEOGRAPHIC SCOPE
FIGURE 3 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: DATA TRIANGULATION
FIGURE 4 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: SNAPSHOT
FIGURE 5 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BOTTOM UP APPROACH
FIGURE 6 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: TOP DOWN APPROACH
FIGURE 7 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: INTERVIEWS BY REGION AND DESIGNATION
FIGURE 8 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: THE CATEGORY VS TIME GRID
FIGURE 10 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET SEGMENTATION
FIGURE 11 INCREASE IN PREVALENCE AND INCIDENCE OF NEUROLOGICAL DISEASES AND DEMAND FOR INTERNAL NEUROSTIMULATION DEVICES AS A ADD ON THERAPY ARE EXPECTED TO DRIVE THE NORTH AMERICA INTERNAL NEUROSTIMULATIOJ DEVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SPINAL CORD STIMULATION IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET IN 2022 TO 2029
FIGURE 13 NORTH AMERICA INCIDENCE OF ISCHEMIA
FIGURE 14 NORTH AMERICA INCIDENCE OF PARKINSON'S DISEASES
FIGURE 15 NORTH AMERICA INCIDENCE OF TREMOR
FIGURE 16 NORTH AMERICA INCIDENCE RATE OF EPILEPSY
FIGURE 17 NORTH AMERICA INCIDENCE RATE OF GASTROPARESIS
FIGURE 18 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET
FIGURE 19 INCIDENCE OF ADULT ONSET BRAIN DISORDERS IN THE U.S. IN 2021
FIGURE 20 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, 2021
FIGURE 21 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, 2022-2029 (USD MILLION)
FIGURE 22 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 23 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 24 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 25 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 26 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 27 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 28 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: SNAPSHOT (2021)
FIGURE 29 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2021)
FIGURE 30 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 31 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 32 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 33 NORTH AMERICA INTERNAL NEUROSTIMULATION DEVICES MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












