Market Analysis and Insights: North America Circulating Tumor Cells (CTC) Liquid Biopsy Market
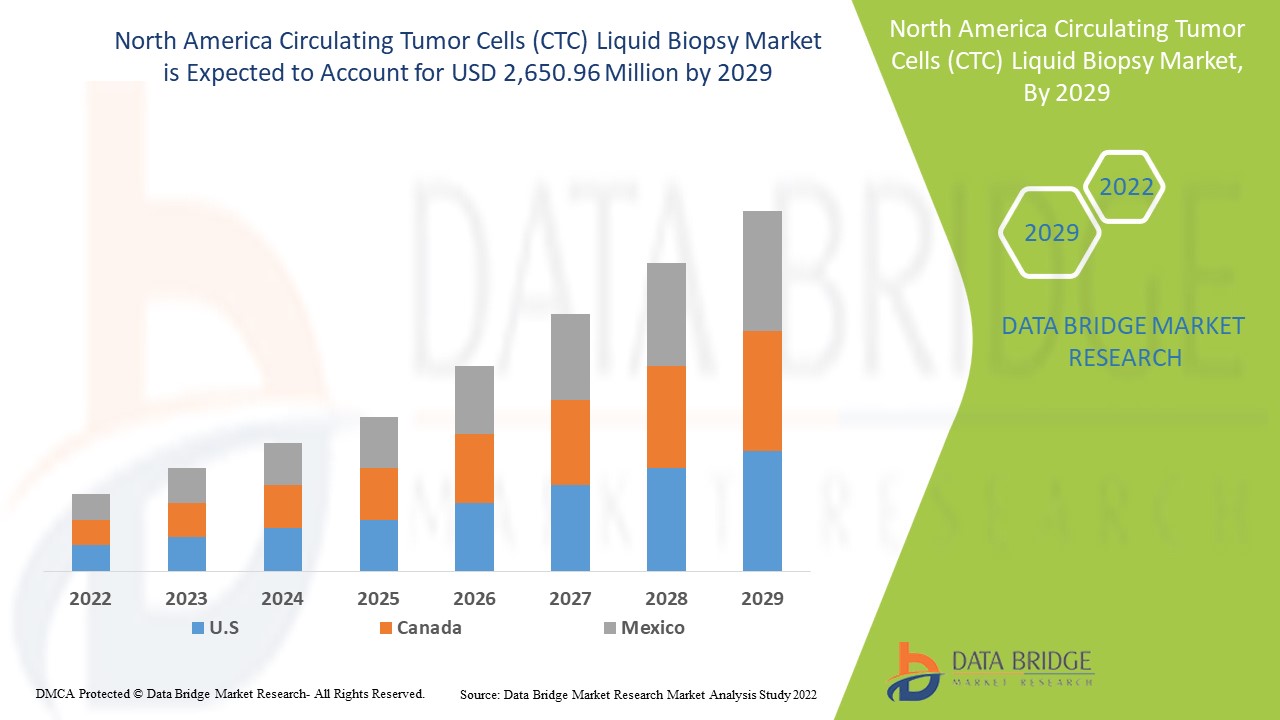
North America circulating tumor cells (CTC) liquid biopsy market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 25.9% in the forecast period of 2022 to 2029 and is expected to reach USD 2,650.96 million by 2029 from USD 442.64 million in 2021. The high prevalence of chronic diseases and increasing R&D activities for its effective application is likely to be the major drivers which propel the demand of the market in the forecast period.
The liquid biopsy is a non-invasive blood test which detects circulating tumor cells and tumor DNA fragments which are released into the blood from primary tumors and metastatic sites. It is a simple and precise alternative to surgical biopsy procedure, which allows surgeon to detect cancer at a very early stage.
The circulating tumor cells are a rare sub-set of cells which function as a seed of metastases. It is found in blood of patients who have developed solid tumors. The testing of circulating tumor cells allows the detection and quantification of tumor cells in the blood of cancer patients. The various types of biological phenotypes of circulating tumor cells (CTCs) exits includes stem cell-like or mixed, mesenchymal or epithelial. These phenotypes are present in blood in a very small quantity. Due to which, their detection needs a phase of isolation-enrichment. After that, a second phase of detection.
The growing demand of circulating tumor cells (CTC) liquid biopsy because of their efficacy, high prevalence of cancer are the major drivers propelling the demand for circulating tumor cells (CTC) liquid biopsy market in the forecast period. However, the unclear regulatory and reimbursement scenario, shortage of skilled personnel is restraining the circulating tumor cells (CTC) liquid biopsy market growth in the forecast period.
North America circulating tumor cells (CTC) liquid biopsy market report provides details of market share, new developments, and impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an Analyst Brief. Our team will help you create a revenue impact solution to achieve your desired goal.
 North America Circulating Tumor Cells (CTC) Liquid Biopsy Market Scope and Market Size
North America Circulating Tumor Cells (CTC) Liquid Biopsy Market Scope and Market Size
North America circulating tumor cells (CTC) liquid biopsy market is categorized into three notable segments which are based on technology, application and end user. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of technology, circulating tumor cells (CTC) liquid biopsy market is segmented into CTC detection methods, CTC enrichment methods, ex vivo positive selection, molecular (RNA)-based technologies, functional in vitro cell invasion assay, xenotransplantation methods, microchips, single spiral microchannel, negative selection and immunocytochemical technologies In 2021, CTC detection methods segment is expected to dominate the market because of increasing use of this technology in the academics and research centers for the tests of liquid biopsy.
- On the basis of application, circulating tumor cells (CTC) liquid biopsy market is segmented into cancer stem cell research, multiple chromosome abnormalities and others. In 2021, cancer stem cell research segment is expected to dominate the market because of increasing demand of early diagnosis and treatment of cancer.
- On the basis of end user, circulating tumor cells (CTC) liquid biopsy market is segmented into research and academic institutes, reference laboratories and hospitals and physician laboratories. In 2021, academic and research institutes segment is expected to dominate the market because of research and academic institutes perform an essential role to accelerate research and development in the various therapeutic area related to liquid biopsies.
North America Circulating Tumor Cells (CTC) Liquid Biopsy Market Country Level Analysis
North America circulating tumor cells (CTC) liquid biopsy market is analysed and market size information is provided by country, technology, application and end user.
The countries covered in the North America circulating tumor cells (CTC) liquid biopsy market report are U.S., Canada and Mexico.
The U.S. is dominating the circulating tumor cells (CTC) liquid biopsy in the North American region due to rise in number of cancer patients and presence of major players in the market.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of sales channels are considered while providing forecast analysis of the country data.
The Presence of Advanced Technology and Strategic Initiatives Taken by Players are Creating New Opportunities in The North America Circulating Tumor Cells (CTC) Liquid Biopsy Market
North America circulating tumor cells (CTC) liquid biopsy market also provides you with detailed market analysis for every country's growth in a particular industry with products sales, the impact of advancement in the market, and changes in regulatory scenarios with their support for the dental instruments market. The data is available for the historic period 2011 to 2020.
Competitive Landscape and North America Circulating Tumor Cells (CTC) Liquid Biopsy Market Share Analysis
North America circulating tumor cells (CTC) liquid biopsy market competitive landscape provide details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width, and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company's focus related to circulating tumor cells (CTC) liquid biopsy market.
The major companies providing circulating tumor cells (CTC) liquid biopsy are Eurofins Genomics ( a subsidiary of Eurofins Scientific), MDx Health, Guardant Health, IMMUCOR, Thermo Fisher Scientific, Inc., Menarini Silicon Biosystems, QIAGEN, Exact Sciences Corporation, Myriad Genetics, Inc., LungLife AI, Inc., Bio-Rad Laboratories, Inc., Illumina, Inc. , Natera Inc., ExoDx ( a subsidiary of Bio-Techne Corporation), Biocept, Inc., F. Hoffman-La Roche Ltd. ,FOUNDATION MEDICINE, INC., Lucence Health, Inc., Inivata Ltd, Biolidics Limited, Vortex Biosciences among others.
For instance,
- In November 2020, Lucence Health, Inc. announced the launch of first early detection study which evaluates the use of its liquid biopsy technology. This will help the company to advance the launch of novel products.
Collaboration, joint ventures, and other strategies by the market player is enhancing the company market in the circulating tumor cells (CTC) liquid biopsy market, which also provides the benefit for the organization to improve their offering for circulating tumor cells (CTC) liquid biopsy market.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TECHNOLOGY LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTERS FIVE FORCES
5 REGULATIONS: NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
5.1 ROLE OF FDA
5.2 ROLE OF CDC AND HCFA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 HIGH PREVALENCE OF CANCER
6.1.2 ADVANTAGES OF LIQUID BIOPSY OVER SURGICAL BIOPSY
6.1.3 GOVERNMENT INITIATIVES TO SPREAD AWARENESS ABOUT EARLY DIAGNOSIS OF CANCER
6.1.4 RISE IN FDA APPROVAL
6.1.5 HEALTHCARE REIMBURSEMENT FOR LIQUID BIOPSY
6.2 RESTRAINTS
6.2.1 DOWNSIDES OF LIQUID BIOPSY
6.2.2 RAPID DEVELOPMENT OF ULTRASENSITIVE IMAGING TECHNOLOGIES SUCH AS MAGNETIC RESONANCE IMAGING
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY THE MARKET PLAYERS
6.3.2 RISE IN HEALTHCARE EXPENDITURE AND DISPOSABLE INCOME
6.3.3 INCREASE IN RESEARCH AND DEVELOPMENT ACTIVITIES
6.3.4 HUGE MARKET POTENTIAL IN DEVELOPING COUNTRIES
6.4 CHALLENGES
6.4.1 SHORTAGE OF SKILLED PERSONNEL
6.4.2 LACK OF ACCESSIBILITY
7 COVID-19 IMPACT ON NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
7.1 IMPACT ON PRICE
7.2 IMPACT ON DEMAND
7.3 IMPACT ON SUPPLY
7.4 KEY INITIATIVES BY MARKET PLAYER DURING COVID 19
7.5 CONCLUSION:
8 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY
8.1 OVERVIEW
8.2 CTC DETECTION METHODS
8.3 CTC ENRICHMENT METHODS
8.4 EX VIVO POSITIVE SELECTION
8.5 MOLECULAR (RNA)-BASED TECHNOLOGIES
8.6 FUNCTIONAL IN VITRO CELL INVASION ASSAY
8.7 XENOTRANSPLANTATION MODELS
8.8 MICROCHIPS
8.9 SINGLE SPIRAL MICROCHANNEL
8.1 NEGATIVE SELECTION
8.11 IMMUNOCYTOCHEMICAL TECHNOLOGIES
9 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 CANCER STEM CELL RESEARCH
9.3 MULTIPLE CHROMOSOME ABNORMALITIES
9.4 OTHERS
10 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER
10.1 OVERVIEW
10.2 RESEARCH & ACADEMIC INSTITUTES
10.3 REFERENCE LABORATORIES
10.4 HOSPITALS AND PHYSICIAN LABORATORIES
11 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION
11.1 NORTH AMERICA
11.1.1 U.S.
11.1.2 CANADA
11.1.3 MEXICO
12 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 GUARDANT HEALTH
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENTS
14.2 EUROFINS GENOMICS (A SUBSIDIARY OF EUROFINS SCIENTIFIC)
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENTS
14.3 FOUNDATION MEDICINE, INC.
14.3.1 COMPANY SNAPSHOT
14.3.2 COMPANY SHARE ANALYSIS
14.3.3 PRODUCT PORTFOLIO
14.3.4 RECENT DEVELOPMENTS
14.4 ILLUMINA, INC.
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENT
14.5 NATERA, INC.
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENT
14.6 BIO-RAD LABORATORIES, INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENT
14.7 QIAGEN
14.7.1 COMPANY SNAPSHOT
14.7.2 REVENUE ANALYSIS
14.7.3 PRODUCT PORTFOLIO
14.7.4 RECENT DEVELOPMENTS
14.8 THERMO FISHER SCIENTIFIC INC.
14.8.1 COMPANY SNAPSHOT
14.8.2 REVENUE ANALYSIS
14.8.3 PRODUCT PORTFOLIO
14.8.4 RECENT DEVELOPMENTS
14.9 BIOCEPT, INC.
14.9.1 COMPANY SNAPSHOT
14.9.2 REVENUE ANALYSIS
14.9.3 PRODUCT PORTFOLIO
14.9.4 RECENT DEVELOPMENT
14.1 BIOLIDICS LIMITED
14.10.1 COMPANY SNAPSHOT
14.10.2 REVENUE ANALYSIS
14.10.3 PRODUCT PORTFOLIO
14.10.4 RECENT DEVELOPMENTS
14.11 EXACT SCIENCES CORPORATION
14.11.1 COMPANY SNAPSHOT
14.11.2 REVENUE ANALYSIS
14.11.3 PRODUCT PORTFOLIO
14.11.4 RECENT DEVELOPMENT
14.12 EXODX (A SUBSIDIARY OF BIO-TECHNE CORPORATION)
14.12.1 COMPANY SNAPSHOT
14.12.2 REVENUE ANALYSIS
14.12.3 PRODUCT PORTFOLIO
14.12.4 RECENT DEVELOPMENTS
14.13 F. HOFFMANN-LA ROCHE LTD
14.13.1 COMPANY SNAPSHOT
14.13.2 RECENT FINANCIALS
14.13.3 PRODUCT PORTFOLIO
14.13.4 RECENT DEVELOPMENTS
14.14 IMMUCOR
14.14.1 COMPANY SNAPSHOT
14.14.2 PRODUCT PORTFOLIO
14.14.3 RECENT DEVELOPMENTS
14.15 INIVATA LTD
14.15.1 COMPANY SNAPSHOT
14.15.2 PRODUCT PORTFOLIO
14.15.3 RECENT DEVELOPMENTS
14.16 LUCENCE HEALTH, INC.
14.16.1 COMPANY SNAPSHOT
14.16.2 PRODUCT PORTFOLIO
14.16.3 RECENT DEVELOPMENTS
14.17 LUNGLIFE AI, INC.
14.17.1 COMPANY SNAPSHOT
14.17.2 PRODUCT PORTFOLIO
14.17.3 RECENT DEVELOPMENTS
14.18 MDXHEALTH
14.18.1 COMPANY SNAPSHOT
14.18.2 REVENUE ANALYSIS
14.18.3 PRODUCT PORTFOLIO
14.18.4 RECENT DEVELOPMENT
14.19 MENARINI SILICON BIOSYSTEMS
14.19.1 COMPANY SNAPSHOT
14.19.2 PRODUCT PORTFOLIO
14.19.3 RECENT DEVELOPMENTS
14.2 MYRIAD GENETICS, INC.
14.20.1 COMPANY SNAPSHOT
14.20.2 REVENUE ANALYSIS
14.20.3 PRODUCT PORTFOLIO
14.20.4 RECENT DEVELOPMENTS
14.21 VORTEX BIOSCIENCES
14.21.1 COMPANY SNAPSHOT
14.21.2 PRODUCT PORTFOLIO
14.21.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
List of Table
TABLE 1 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 2 NORTH AMERICA CTC DETECTION METHODS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA CTC ENRICHMENT METHODS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA EX VIVO POSITIVE SELECTION IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA MOLECULAR (RNA)-BASED TECHNOLOGIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA FUNCTIONAL IN VITRO CELL INVASION ASSAY IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA XENOTRANSPLANTATION MODELS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA MICROCHIPS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA SINGLE SPIRAL MICROCHANNEL IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA NEGATIVE SELECTION IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA IMMUNOCYTOCHEMICAL TECHNOLOGIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CANCER STEM CELL RESEARCH IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA MULTIPLE CHROMOSOME ABNORMALITIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA OTHERS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA RESEARCH & ACADEMIC INSTITUTES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA REFERENCE LABORATORIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA HOSPITALS AND PHYSICIAN LABORATORIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 24 U.S. CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 25 U.S. CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 26 U.S. CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 27 CANADA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 28 CANADA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 29 CANADA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 30 MEXICO CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 31 MEXICO CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 32 MEXICO CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET : NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: SEGMENTATION
FIGURE 11 THE HIGH PREVALENCE OF CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 CTC DETECTION METHODS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
FIGURE 15 PREVALENCE OF BREAST CANCER IN EUROPE, INDIA, AND IN THE U.S.
FIGURE 16 PREVALENCE OF LUNG CANCER IN VARIOUS COUNTRIES, WITH HUNGARY BEING THE HIGHEST PREVALENCE RATE IN WOMEN AND MEN
FIGURE 17 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, 2021
FIGURE 18 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, 2020-2029 (USD MILLION)
FIGURE 19 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, CAGR (2022-2029)
FIGURE 20 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 21 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, 2021
FIGURE 22 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, 2020-2029 (USD MILLION)
FIGURE 23 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 24 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 25 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, 2021
FIGURE 26 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 27 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, CAGR (2022-2029)
FIGURE 28 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, LIFELINE CURVE
FIGURE 29 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: SNAPSHOT (2021)
FIGURE 30 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY COUNTRY (2021)
FIGURE 31 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY COUNTRY (2022 & 2029)
FIGURE 32 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY COUNTRY (2021 & 2029)
FIGURE 33 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY (2022-2029)
FIGURE 34 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.