North America Cervical Cancer Diagnostic Market Analysis and Insights
The North America cervical cancer diagnostic market is growing in the forecast year due to the rise in market players and the availability of various cervical cancer diagnostic products and brands. Along with this, market players are engaged in advanced cervical cancer diagnostics. The rising prevalence of cervical cancer is further expected to boost market growth. However, the stringent rules and regulations may restrain market growth in the forecast period. The various government and private collaborations, increasing R&D activities, and strategic initiatives by market players are giving opportunities to the market. However, false results in cervical cancer screening tests are projected to be a key challenge to market growth.
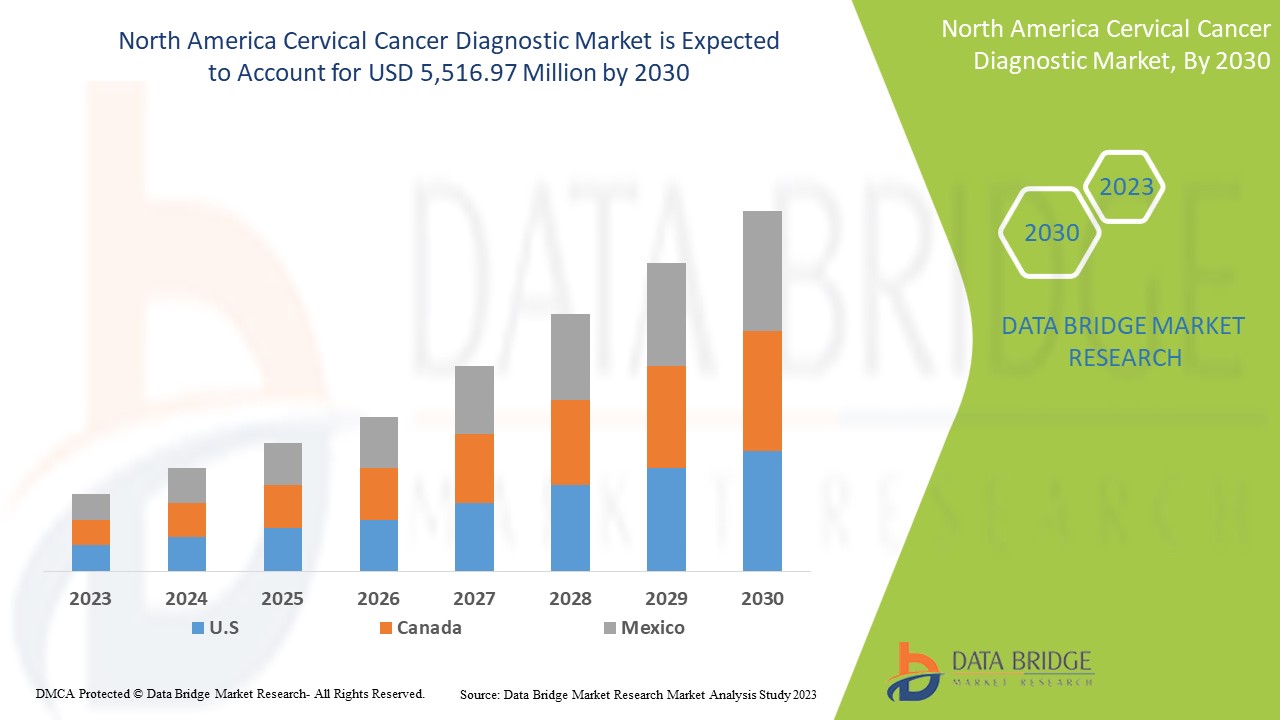
The North America cervical cancer diagnostic market is expected to gain market growth in the forecast period of 2023 to 2030. Data Bridge Market Research analyses that the market is growing with a CAGR of 7.0% in the forecast period of 2023 to 2030 and is expected to reach USD 5,516.97 million by 2030.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customisable to 2020-2015) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Product Type (Imaging Test, Screening Test, Visual Examination, Cervical Biopsies, and Other Procedures), Age Group (Below 21, 21-29, 30-65, 65 and Above), Stages (Stage I, Stage II, Stage III, Stage IV), End Users (Hospitals, Diagnostic Laboratories, Specialty Clinics, Community Health Centers, Cancer Research Organization, Cancer and Radiation Therapy Centers), Distribution Channel (Direct Tender, Retail Sales, Online Sales) |
|
Countries Covered |
U.S., Canada, Mexico |
|
Market Players Covered |
Siemens Healthcare GmbH, BD, F. Hoffmann-La Roche Ltd, Abbott, Hologic, Inc., Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., QIAGEN, The Cooper Companies Inc., Seegene Inc., Sysmex Corporation, MobileODT, Zilico, Jiangsu Mole Bioscience Co. Ltd., Guided Therapeutics, Inc., GenomeMe Lab Inc., Arbor Vita Corporation, and LCM GENECT Srl among others |
Market Definition:
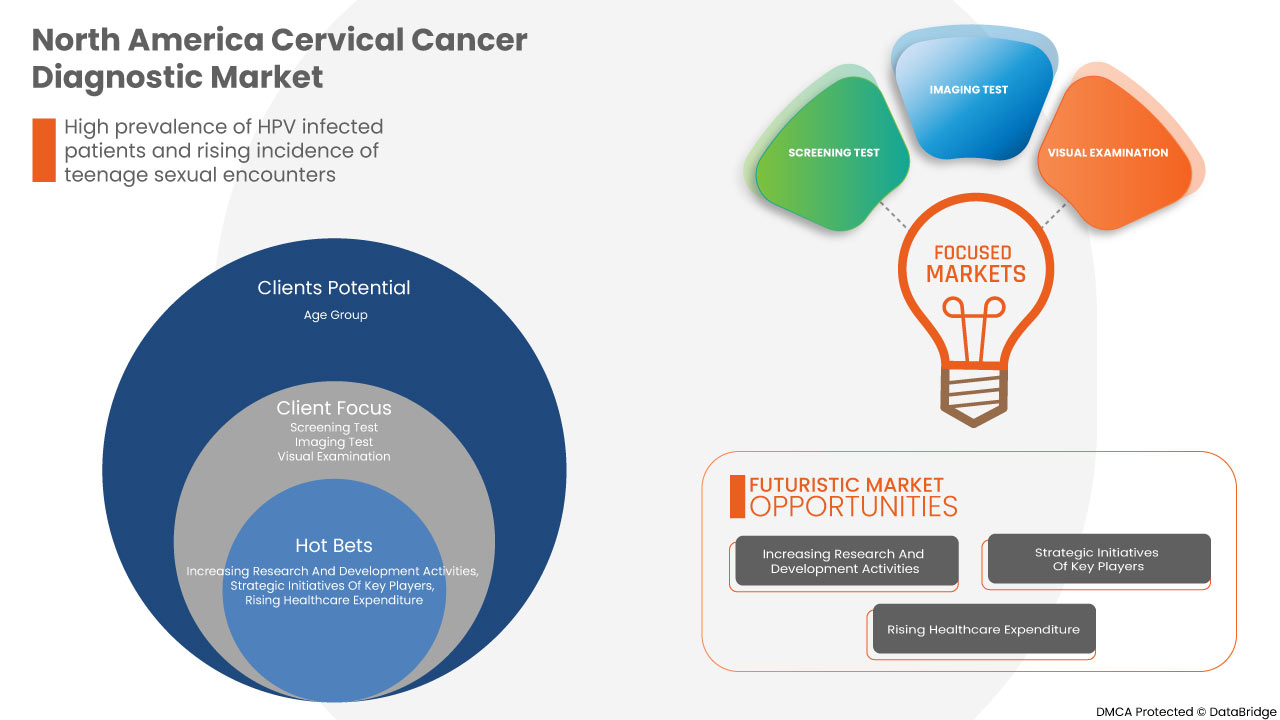
Cervical cancer is a type of cancer that develops in the cervix of the female reproductive tract. It is becoming more prevalent as the number of HPV-infected patients increases, and there is a greater emphasis on early detection and treatment, which is expected to accelerate the development of cervical cancer diagnosis. Increasing government investment in raising awareness about early cancer detection and increasing healthcare spending would also propel business growth. The most common cause of cervical cancer is HPV (Human Papillomavirus) infection. Other lifestyle choices that can raise the risk include smoking, drinking, a diet low in fruits, and vegetables, taking birth control pills, and teenage sexual encounters. Since, cervical cancer is treatable once diagnosed in an early stage, women at risk of developing the disease must undergo routine testing to identify the disease early, allowing the market to expand.
North America Cervical Cancer Diagnostic Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
-
Rising awareness of the early diagnosis of cervical cancer
There is an enormous range of risk factors being reported for cervical cancer. Therefore awareness about its diagnosis has increased in recent years. Various diagnostic tests are available, such as PAP (Papanicolaou) testing, human papillomavirus testing, colposcopy, cervical biopsies, and cystoscopy among others. Hence, to reduce the risk factors, early diagnosis is very important.
-
Increasing prevalence and incidence of cervical cancer
Cervical cancer is a type of cancer that develops in the female reproductive tract's cervix. The irregular development of cancer cells in the cervix tissue frequently defines cervical cancer. Adenocarcinoma or squamous cell carcinoma can develop from cervical cancer. The most common cause of cervical cancer is HPV (Human Papillomavirus) infection. Cervical cancer is classified into two types' adenocarcinoma and squamous cell carcinoma. Cervical cancer is diagnosed using a variety of advanced laboratory tests, tools, and procedures that evaluate abnormal cells and strains of the human papillomavirus (HPV).
Opportunities
-
Rising healthcare expenditure
Healthcare expenditure has increased worldwide as people's disposable income in various countries increases. Moreover, to accomplish the population requirements, government bodies and healthcare organizations are taking the initiative to accelerate healthcare expenditure. The rise in healthcare expenditure simultaneously helps healthcare settings to improve their treatment facilities on cervical cancer diagnostic as the disorder has been highly prevalent in recent years.
Also, the strategic initiatives key market players take will provide structural integrity and future opportunities for the North America cervical cancer diagnostic market in the forecast period.
Restraints/Challenges
However, cervical cancer diagnostic drugs may have adverse side effects; it is challenging to balance the risks with the benefits of treatment due to the increasing side effects of cancer medication hampering the market demand.
Moreover, increasing approvals of HPV vaccines by regulatory authorities are expected to hinder the growth of the North America cervical cancer diagnostic market. These developments are rapidly happening across the globe to reduce the cases of cervical cancer, which can be a restraining factor for the market.
The use of various treatment drugs across the globe is rapidly increasing, and with the increasing prevalence of cervical cancer, there is a need for timely diagnosis and treatment. At the same time, the players of the cervical cancer diagnostic manufacturers in the market have to follow certain regulations to get approval from the upper authorities for launching the product in the market. These stringent guidelines need to be followed; this is one of the most difficult tasks of all the steps. The pre-market approval of various medical drugs varies from one country to another.
Recent Development
- In August 2020, Siemens Healthcare GmbH agreed with Varian Medical Systems, Inc. for acquisition; with this acquisition, Siemens Healthcare has helped develop advanced solutions to treat cancer and strengthen its position in the healthcare industry
North America Cervical Cancer Diagnostic Market Scope
The North America cervical cancer diagnostic market is segmented into product type, age group, stages, end users, and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Product Type
- Imaging Test
- Screening Test
- Visual Examination
- Cervical Biopsies
- Other Procedures
On the basis of product type, the North America cervical cancer diagnostic market is segmented into imaging test, screening test, visual examination, cervical biopsies, and other procedures.
Age Group
- Below 21
- 21-29
- 30-65
- 65 and Above
On the basis of age group, the North America cervical cancer diagnostic market is segmented into below 21, 21-29, 30-65, and 65 and above.
Stages
- Stage I
- Stage II
- Stage III
- Stage IV
On the basis of stage, the North America cervical cancer diagnostic market is segmented into stage I, stage II, stage III, and stage IV.
End Users
- Cancer And Radiation Therapy Centers
- Hospitals
- Specialty Clinics
- Cancer Research Organization
- Diagnostic Laboratories
- Community Health Centers
On the basis of end users, the North America cervical cancer diagnostic market is segmented into hospitals, diagnostic laboratories, specialty clinics, community health centers, cancer research organization, and cancer and radiation therapy centers.
Distribution Channel
- Direct Tender
- Retail Sales
- Online Sales

On the basis of distribution channel, the North America cervical cancer diagnostic market is segmented into direct tender, retail sales, and online sales.
Cervical Cancer Diagnostic Market Regional Analysis/Insights
The North America cervical cancer diagnostic market is analysed, and market size insights and trends are provided by country, product type, age group, stages, end users, and distribution channel as referenced above.
Some countries covered In the North America cervical cancer diagnostic market are the U.S., Canada, and Mexico.
The U.S. is expected to dominate the North America cervical cancer diagnostic market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the rising awareness of the early diagnosis of cervical cancer.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of North American brands and their challenges faced due to high competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
North America Competitive Landscape and Cervical Cancer Diagnostic Market Share Analysis
The North America cervical cancer diagnostic market competitive landscape provides details of the competitors. Details include company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points are only related to the companies focusing on the North America cervical cancer diagnostic market.
Some of the major players operating in the North America cervical cancer diagnostic market are Siemens Healthcare GmbH, BD, F. Hoffmann-La Roche Ltd, Abbott, Hologic, Inc., Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., QIAGEN, The Cooper Companies Inc., Seegene Inc., Sysmex Corporation, MobileODT, Zilico, Jiangsu Mole Bioscience Co. Ltd., Guided Therapeutics, Inc., GenomeMe Lab Inc., Arbor Vita Corporation, and LCM GENECT Srl among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
4.3 INDUSTRY INSIGHTS:
4.3.1 CERVICAL CANCER DIAGNOSIS
4.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND INCIDENCE OF CERVICAL CANCER
6.1.2 RISING AWARENESS OF EARLY DIAGNOSIS OF CERVICAL CANCER
6.1.3 HIGH PREVALENCE OF HPV-INFECTED PATIENTS AND RISING INCIDENCE OF TEENAGE SEXUAL ENCOUNTERS
6.2 RESTRAINTS
6.2.1 DEVELOPMENT IN THE FIELD OF HPV VACCINE
6.2.2 SIDE EFFECTS OF TREATMENT DRUGS
6.3 OPPORTUNITIES
6.3.1 RISING HEALTHCARE EXPENDITURE
6.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.4 CHALLENGES
6.4.1 STRINGENT RULES AND REGULATIONS
6.4.2 FALSE RESULTS IN SCREENING TESTS AND UNAVAILABILITY OF IMPROVED HEALTHCARE INFRASTRUCTURE
7 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 SCREENING TEST
7.2.1 HPV TEST
7.2.1.1 ASSAYS
7.2.1.2 KITS/REAGENTS
7.2.2 PAP TEST
7.2.2.1 ASSAYS
7.2.2.2 KITS/REAGENTS
7.2.3 BLOOD TEST
7.3 IMAGING TEST
7.3.1 PET CT-SCAN
7.3.2 MAGNETIC RESONANCE IMAGING (MRI)
7.3.3 ULTRASOUND
7.3.4 X-RAY
7.3.5 OTHERS
7.4 CERVICAL BIOPSIES
7.4.1 LIQUID BIOPSY
7.4.2 ENDOCERVICAL CURETTAGE
7.4.3 COLPOSCOPIC BIOPSY
7.5 VISUAL EXAMINATION
7.5.1 CYSTOSCOPY
7.5.2 SIGMOIDOSCOPY
7.6 OTHER PROCEDURES
8 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP
8.1 OVERVIEW
8.2 30-65
8.3 65 AND ABOVE
8.4 21-29
8.5 BELOW 21
9 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES
9.1 OVERVIEW
9.2 STAGE I
9.3 STAGE II
9.4 STAGE III
9.5 STAGE IV
10 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER
10.1 OVERVIEW
10.2 CANCER AND RADIATION THERAPY CENTERS
10.3 HOSPITALS
10.4 SPECIALTY CLINICS
10.5 CANCER RESEARCH ORGANIZATION
10.6 DIAGNOSTIC LABORATORIES
10.7 COMMUNITY HEALTH CENTERS
11 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 RETAIL SALES
11.4 ONLINE SALES
12 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 SIEMENS HEALTHCARE GMBH
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENT
15.2 BD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 F. HOFFMANN- LA ROCHE LTD
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 ABBOTT
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 HOLOGIC, INC.
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.6 ARBOR VITA CORPORATION
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 BIO-RAD LABORATORIES, INC.
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 GENOMEME LAB INC.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 GUIDED THERAPEUTICS, INC
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENT
15.1 JIANGSU MOLE BIOSCIENCE CO., LTD.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENTS
15.11 LCM GENECT SRL
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 MOBILEODT
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.13 QIAGEN
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 QUEST DIAGNOSTICS INCORPORATED
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SEEGENE INC.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
15.16 SYSMEX CORPORATION
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 THE COOPER COMPANIES INC
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 ZILICO
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
TABLE 1 CERVICAL CANCER CAN BE DIAGNOSED BY VARIOUS TESTS AND BIOPSIES, AS LISTED BELOW:
TABLE 2 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA OTHER PROCEDURES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA 30-65 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA 65 AND ABOVE IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA 21-29 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA BELOW 21 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA STAGE I IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA STAGE II IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA STAGE III IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA STAGE IV IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA CANCER AND RADIATION THERAPY CENTERS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA HOSPITALS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA SPECIALTY CLINICS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA CANCER RESEARCH ORGANIZATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA DIAGNOSTIC LABORATORIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA COMMUNITY HEALTH CENTERS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA DIRECT TENDER IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA RETAIL SALES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA ONLINE SALES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 47 U.S. CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 U.S. SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 49 U.S. HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 50 U.S. PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 U.S. IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 52 U.S. CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 53 U.S. VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 54 U.S. CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 55 U.S. CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 56 U.S. CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 57 U.S. CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 58 CANADA CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 CANADA SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 CANADA HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 CANADA PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 CANADA IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 CANADA CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 CANADA VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 65 CANADA CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 66 CANADA CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 67 CANADA CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 68 CANADA CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 69 MEXICO CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 70 MEXICO SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 71 MEXICO HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 72 MEXICO PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 73 MEXICO IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 74 MEXICO CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 MEXICO VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 MEXICO CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 77 MEXICO CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 78 MEXICO CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 79 MEXICO CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE AND INCIDENCE OF CERVICAL CANCER ARE EXPECTED TO DRIVE THE NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET IN THE FORECAST PERIOD
FIGURE 12 SCREENING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET
FIGURE 14 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP 2022
FIGURE 19 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, LIFELINE CURVE
FIGURE 22 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, 2022
FIGURE 23 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY STAGES, LIFELINE CURVE
FIGURE 26 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, 2022
FIGURE 27 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET : BY END USER, LIFELINE CURVE
FIGURE 30 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: SNAPSHOT (2022)
FIGURE 35 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2022)
FIGURE 36 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2023 & 2030)
FIGURE 37 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2030)
FIGURE 38 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: PRODUCT TYPE (2023-2030)
FIGURE 39 NORTH AMERICA CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












