Market Analysis and Insights
Chronic diseases-including cancer, musculoskeletal disorders, neurology disorders, chronic injuries, cardiovascular and gastrointestinal -can lead to hospitalization, long-term disability, reduced quality of life, and death.
The mesenchymal stem cells penetrate and integrate into multiple organs, repair cardiovascular, lung, and spinal cord injuries, and improve the state of autoimmune diseases, liver, and bone and cartilage diseases. Stems cells are a potent tool for the treatment of infections caused by inflammation, immune system failure, and, or tissue degeneration
The drivers responsible for the growth of the Middle East and Africa stem cell therapy market are the increased incidence of chronic diseases, rise in GMP-certification approvals for cell therapy production facilities, growing biotechnology sector and rise in clinical trials for stem-cell-based therapies. However, factors that are expected to restrain the market growth are the rise in the cost of stem cell-based research, and the risks faced while undergoing stem cell therapy, and the availability of alternatives.
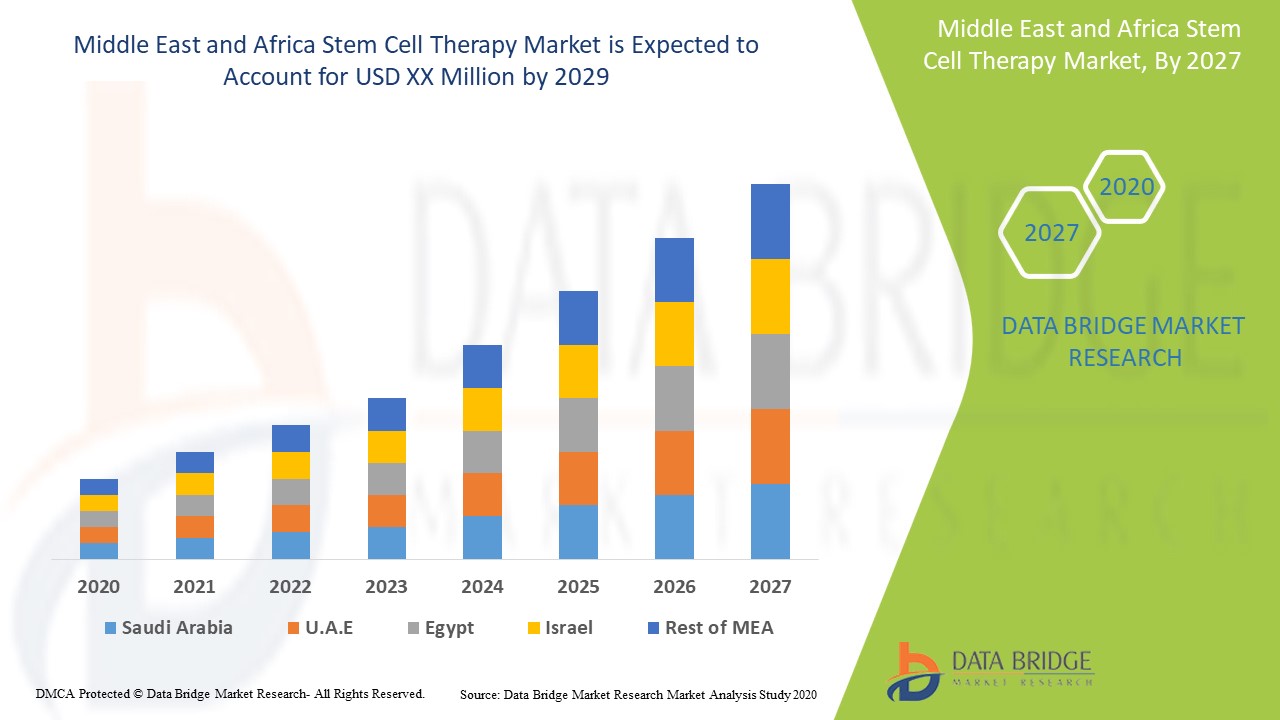
The Middle East and Africa stem cell therapy is supportive and aims to reduce the severity of the symptoms. Data Bridge Market Research analyses that the Middle East and Africa stem cell therapy market is expected to reach the value of USD 1.56 million and grow at a CAGR of 4.1% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Year |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Product Type (Bone Marrow Derived Mesenchymal Cells, Placental or Umbilical Stem Cell, Adipose Tissue Derived Mesenchymal Stem Cells, and Others), Type (Allogenic Stem Cell Therapy and Autologous Stem Cell Therapy), Application (Musculoskeletal Disorders, Acute Graft-Versus-Host Disease (AGVHD), Wounds and Injuries, Cardiovascular Diseases, Surgeries, Gastrointestinal Diseases, and Others), End User (Hospitals and Surgical Centers, Therapeutic Companies, Services Companies, and Others), Distribution Channel (Direct Tender, Third Party Distributors) |
|
Countries Covered |
Israel |
|
Market Players Covered |
U.S. Stem Cell, Inc., STEMPEUTICS RESEARCH PVT LTD, Pluristem Inc., among others |
Market Definition
Stem cells are the body's initial materials from which all other cells with specialized functions are generated. Under the right conditions in the body or a laboratory, stem cells divide to form more cells called daughter cells. The daughter cells become new stem cells or specialized cells (differentiation) with a more specific function, such as blood cells, brain cells, heart muscle cells, or bone cells. Much interest in stem cells has brought interest among the research scientists. Understanding how a disease develops and occurs by using stem cells, generating healthy cells to replace cells, and testing novel drug safety and efficacy are the scientific reasons why stem cell therapeutics are used.
Stem cell therapy promotes dysfunctional or injured tissue repair response using stem cells or derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply. The adult stem cells, such as adipose tissue-derived mesenchymal stem cells, bone marrow-derived mesenchymal stem cells, and placental or umbilical stem cells, are found in small numbers in most tissues. The embryonic stem cells originate from embryos, which are three to five days old. Emerging indications indicate that adult stem cells may be able to create various types of cells.
Middle East and Africa Stem Cell Therapy Market Dynamics
Drivers
- Rise in prevalence and incidence of chronic diseases
Chronic diseases are common health conditions around the world. In the Middle East and Africa one in three adults suffers from chronic conditions. Chronic diseases have affected the health and quality of life of many citizens. Chronic diseases—including cancer, musculoskeletal disorders, neurology disorders, chronic injuries, cardiovascular and gastrointestinal —can lead to hospitalization, long-term disability, reduced quality of life, and death.
The mesenchymal stem cells penetrate and integrate into multiple organs, repair cardiovascular, lung and spinal cord injuries, and improve the state of autoimmune diseases, liver, and bone and cartilage diseases. Stems cells are a potent tool for the treatment of diseases caused by inflammation, immune system failure, and or tissue degeneration
For instance,
- In 2021, the World Health Organization (WHO) data, approximately 1.7 billion people were down with musculoskeletal disorders worldwide. Low back pain causes a high burden of musculoskeletal disorders
- Rise in investment in research and development
The stem cell research is funded by the National Institute of Health (NIH) budget. The private sector also funds stem cell research, but such investment generally occurs later, during the testing and development phase, then during initial basic research. With stem cell therapies being a new field, an unbiased governmental body must supervise them. The FDA is cautious and thorough, but they are endlessly struggling for funding, making a long-term investment that aligns the payment with the potential future beneficiaries.
- Growing biotechnology sector
People are getting more aware of their health, and also there is alertness for preventive healthcare. Emphasis on healthcare is gaining popularity. Awareness of disease and symptoms is essential for screening and early detection of infections due to advanced technology available in the healthcare system, such as stem cell technology which is helping the healthcare providers lead to higher survival rates. A large number of biotechnology companies focusing on the development of stem cell-based therapies are expected to drive the market's growth.
For instance,
- In 2018, according to Centres for Medicare and Medicaid Services, NHE rose 4.6% to USD 3.60 trillion (USD 11,172.00 per person), accounting for 17.7% of GDP. National health spending will reach USD 6.20 trillion by 2028, with an average annual rate of 5.4% for 2019-2028
Opportunities
- The rise in healthcare expenditure
Moreover, the rise in the research and development activities and increasing investments by government and private organizations will boost new opportunities for the market's growth rate.
For instance,
• In 2019, according to Knoema, the health expenditure per capita for Israel was USD 3,456. Health expenditure per capita in Israel increased from USD 1,514 in 2000 to USD 3,456 in 2019, growing at an average annual rate of 4.66%
- Strategic initiative by market players
The demand for stem cell therapy has increased due to the timely treatment of chronic conditions. These favorable factors enhance the need for medications, and to achieve the market demand, minor and major market players are utilizing various strategies.
The major players are also trying to devise specific strategies, such as product launches, acquisitions, approvals, expansions, and partnerships, to ensure the smooth running of the business, avoid risks, and increase the long-term growth in the sales of the market.
For instance,
- In March 2022, Pluristem Inc. announced the positive phase I clinical trials for PLX-R18. The U.S. Food and Drug Administration (FDA) granted orphan drug designation to treat graft failure and incomplete hematopoietic recovery following HCT and the prevention and treatment of acute radiation syndrome (ARS). The phase I clinical trials completed would result in the market player being qualified to conduct Phase II and III clinical trials and post-marketing approval
These strategic initiatives by the market players, including acquisition, conferences, and focused segment product launches, are helping the companies grow and improve the company's product portfolios, ultimately leading to more revenue generation. Hence, these strategic initiatives by the market players provide an opportunity that is helping them to drive market growth.
Restraints/Challenges
- The rise in cost of stem-cell based therapy research
Stem cell therapy is a developing and novel treatment option for the treatment of several disorders. Sometimes, the cost of the therapy is a concern for several conditions. The stem-cell therapy treatment procedures. The stem cell field is still highly specialized and has not been adopted by the mainstream and insurance companies. The cost of stem cell therapy-based research therapy is not covered by medical insurance. These costs are pushed on patients. Therefore, the present high cost is expected to show a descending trend.
For instance,
- In 2022, the data by the Bioinformant states that the cost of autologous stem cell therapy in the U.S. is around USD 150,000
- Lack of skilled professionals
The lack or shortage of skilled expertise would challenge the pace of recovery and growth in one place. Often, the unemployed people in one place have skills that are in short supply elsewhere. Moreover, rapid technological advancement in this field also leads to a lack of expertise.
Stem cell therapy encompasses a modern technology to replace damaged cells with healthy new ones. Nowadays, Stem cell therapies provide substantial benefits to patients suffering from a wide range of diseases such as neuronal disease, diabetes, and injuries such as brain trauma and spinal cord injuries.
The hematologists dealing in stem cell therapies must have received proper stem cell therapy administration training. They must be familiar with the on-site well-organized system for specific emergency administration of treatments. The stem cell therapy coordinator ensures that all relevant personnel must be informed when a patient receives therapy. The coordinator can be the health professionals, including state registered nurses.
The Middle East and Africa stem cell therapy market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Middle East and Africa stem cell therapy market, contact Data Bridge Market Research for an Analyst Brief; our team will help you make an informed market decision to achieve market growth.
Patient Epidemiology Analysis
In 2017, the data by the Middle East and Africa Burden of Disease (GBD) stated that lower back pain caused the maximum number of cases, followed by other musculoskeletal disorders (21.5%),
The Middle East and Africa stem cell therapy market also provides you with detailed market analysis for patient analysis, prognosis, and cures. Prevalence, incidence, mortality, and adherence rates are some of the data variables available in the report. Direct or indirect impact analyses of epidemiology to the market growth are analyzed to create a more robust and cohort multivariate statistical model for forecasting the market during the growth period.
Impact of COVID-19 on the Middle East and Africa Stem Cell Therapy Market
During the pandemic, stem cell therapy had a remarkable effect on reducing mortality and morbidity of patients with COVID-19. Further large-scale studies are needed to approve these results. A protocol for stem cell therapy in COVID-19 infection should be defined to achieve the best possible clinical outcomes. Clinical trials were conducted during COVID-19.
Recent Development
- In March 2022, Pluristem Inc. announced the positive phase I clinical trials for PLX-R18. The U.S. Food and Drug Administration (FDA) granted orphan drug designation to treat graft failure and incomplete hematopoietic recovery following HCT and the prevention and treatment of acute radiation syndrome (ARS). The phase I clinical trials completed would result in the market player being qualified to conduct Phase II and III clinical trials and post marketing approval.
Middle East and Africa Stem Cell Therapy Market Scope
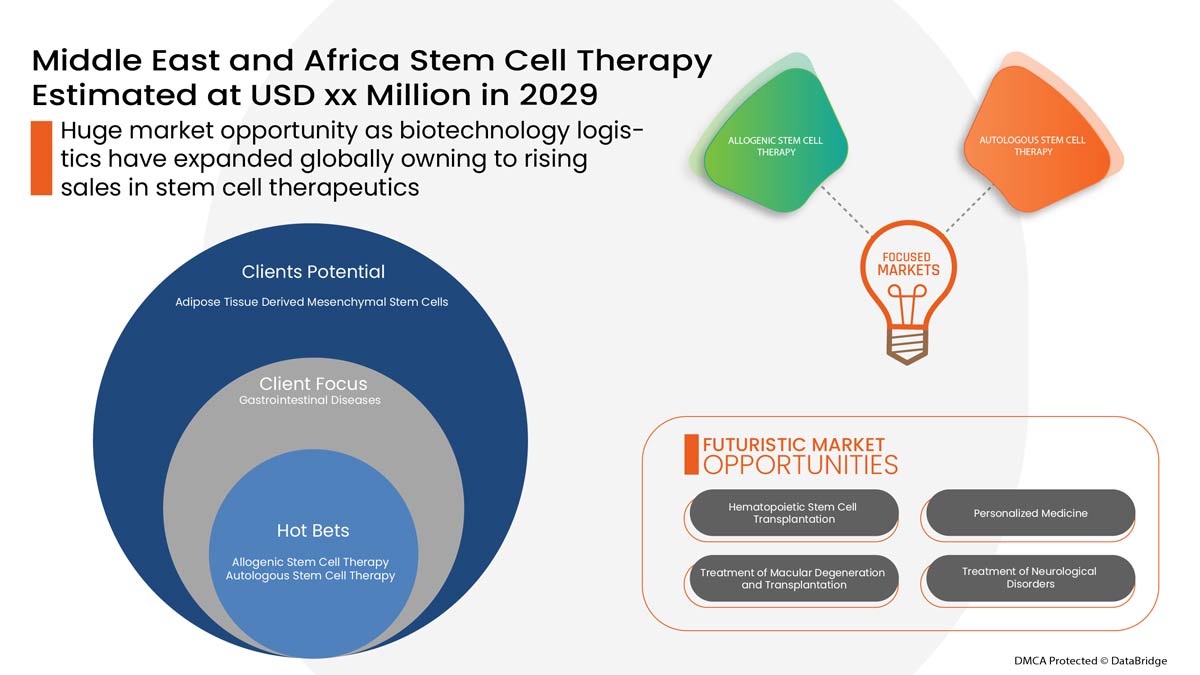
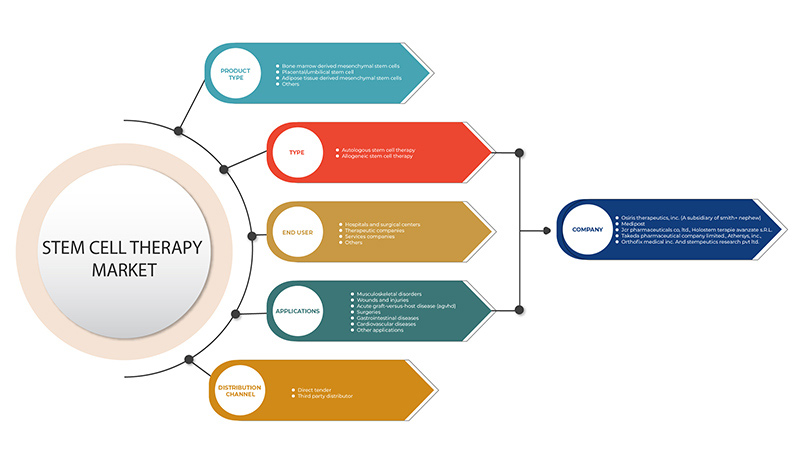
The Middle East and Africa stem cell therapy market is segmented into five segments based on product type, type, application, end user, and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Bone Marrow Derived Mesenchymal Stem Cells
- Placental or Umbilical Stem Cell
- Adipose Tissue Derived Mesenchymal Stem Cells
- Others
On the basis of product type, the Middle East and Africa stem cell therapy market is segmented into bone marrow derived mesenchymal stem cells, placental or umbilical stem cell, adipose tissue derived mesenchymal stem cells, and others.
Type
- Allogeneic Stem Cell Therapy
- Autologous Stem Cell Therapy
On the basis of type, the Middle East and Africa stem cell therapy market is segmented into allogenic stem cell therapy, and autologous stem cell therapy.
Application
- Musculoskeletal Disorders
- Wounds and Injuries
- Acute Graft-Versus-Host Disease (AGVHD)
- Surgeries
- Gastrointestinal Diseases
- Cardiovascular Diseases
- Others
On the basis of application, the Middle East and Africa stem cell therapy market is segmented into musculoskeletal disorders, wounds and injuries, acute graft-versus-host disease (AGVHD), surgeries, gastrointestinal diseases, cardiovascular diseases, and others.
End User
- Hospitals and Surgical Centers
- Therapeutic Companies
- Services Companies
- Others
On the basis of end user, the Middle East and Africa stem cell therapy market is segmented into hospitals and surgical centers, therapeutic companies, services companies, and others.
Distribution Channel
- Direct Tenders
- Third Party Distributors
On the basis of distribution channel, the Middle East and Africa stem cell therapy market is segmented into direct tender, and third party distributors.
Middle East and Africa Stem Cell Therapy Market Regional Analysis/Insights
The Middle East and Africa stem cell therapy market is analyzed, and market size insights and trends are provided by regions, product type, type, application, end user, and distribution channel, as referenced above.
One of the countries covered in the Middle East and Africa stem cell therapy market report is Israel. Israel is expected to dominate the market due to a rise in chronic diseases and increased healthcare expenditure.
The country section of the report also provides individual market impacting factors and changes in regulations in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of the Middle East and African brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East and Africa Stem Cell Therapy Market Share Analysis
The Middle East and Africa stem cell therapy market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, the Middle East and Africa presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the Middle East and Africa stem cell therapy market.
Some of the major players operating in the Middle East and Africa stem cell therapy market are U.S. Stem Cell, Inc., STEMPEUTICS RESEARCH PVT LTD, Pluristem Inc., among others.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or can drop down your inquiry. The key research methodology used by the DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Middle East and Africa versus Regional, and Vendor Share Analysis. To know more about the research methodology, drop an inquiry to speak to our industry experts.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR THE MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET
7 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 THE RISE IN PREVALENCE AND INCIDENCE OF CHRONIC DISEASES
8.1.2 RISE IN INVESTMENT IN RESEARCH AND DEVELOPMENT AND AVAILABILITY OF FUNDING FOR STEM CELL RESEARCH
8.1.3 GROWING BIOTECHNOLOGY SECTOR
8.1.4 RISE IN GMP CERTIFICATION APPROVALS FOR CELL THERAPY PRODUCTION FACILITIES
8.1.5 RISE IN CLINICAL TRIALS FOR STEM-CELL-BASED THERAPIES
8.2 RESTRAINTS
8.2.1 THE RISE IN COST OF STEM-CELL-BASED THERAPY RESEARCH
8.2.2 THE RISKS FACED WHILE UNDERGOING STEM CELL THERAPY
8.2.3 ETHICAL CONCERNS RELATED TO STEM CELL THERAPY RESEARCH
8.2.4 AVAILABILITY OF ALTERNATIVES
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVE BY MARKET PLAYERS
8.3.2 RISE IN HEALTHCARE EXPENDITURE
8.3.3 THE EMERGENCE OF INDUCED PLURIPOTENT STEM CELLS (IPSCS)
8.4 CHALLENGES
8.4.1 THE LACK OF SKILLED PROFESSIONALS REQUIRED FOR STEM CELL THERAPY
8.4.2 STRINGENT REGULATIONS
9 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 BONE MARROW DERIVED MESENCHYMAL STEM CELLS
9.3 PLACENTAL/UMBILICAL STEM CELL
9.4 ADIPOSE TISSUE DERIVED MESENCHYMAL STEM CELLS
9.5 OTHERS
10 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY TYPE
10.1 OVERVIEW
10.2 ALLOGENEIC STEM CELL THERAPY
10.2.1 MUSCULOSKELETAL DISORDERS
10.2.2 WOUNDS AND INJURIES
10.2.3 ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD)
10.2.4 SURGERIES
10.2.5 GASTROINTESTINAL DISEASES
10.2.6 OTHER APPLICATION
10.3 AUTOLOGOUS STEM CELL THERAPY
10.3.1 CARDIOVASCULAR DISEASES
10.3.2 GASTROINTESTINAL DISEASES
10.3.3 OTHER APPLICATION
11 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 MUSCULOSKELETAL DISORDERS
11.3 WOUNDS AND INJURIES
11.4 ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD)
11.5 SURGERIES
11.6 GASTROINTESTINAL DISEASES
11.7 CARDIOVASCULAR DISEASES
11.8 OTHER APPLICATION
12 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL AND SURGICAL CENTERS
12.3 THERAPEUTIC COMPANIES
12.4 SERVICES COMPANIES
12.5 OTHERS
13 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 THIRD PARTY DISTRIBUTORS
14 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY REGION
14.1 MIDDLE EAST AND AFRICA
14.1.1 ISRAEL
15 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 OSIRIS THERAPEUTICS, INC. (A SUBSIDIARY OF SMITH+NEPHEW) (2021)
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 JCR PHARMACEUTICALS CO., LTD ( (2021)
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 ORTHOFIX MEDICAL INC. (2021)
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.4 MEDIPOST (2021)
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS
17.5 TAKEDA PHARMACEUTICAL COMPANY LIMITED (2021)
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 CORESTEM, INC. (2021)
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 PHARMICELL CO., LTD. (2021)
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.8 ANTEROGEN.CO., LTD (2021)
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENTS
17.9 ATHERSYS, INC.(2021)
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENTS
17.1 BRAINSTORM CELL LIMITED (2021)
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENTS
17.11 BIORESTORATIVE THERAPIES, INC. (2021)
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENTS
17.12 HOLOSTEM TERAPIE AVANZATE S.R.L. (2021)
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENTS
17.13 INTERNATIONAL STEMCELL CORPORATION (2021)
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 MESOBLAST LTD (2021)
17.14.1 COMPANY SNAPSHOT
17.14.2 REVENUE ANALYSIS
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENTS
17.15 PLURISTEM INC.(2021)
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 STEMPEUTICS RESEARCH PVT LTD
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENTS
17.17 U.S. STEM CELL, INC. (2021)
17.17.1 COMPANY SNAPSHOT
17.17.2 REVENUE ANALYSIS
17.17.3 PRODUCT PORTFOLIO
17.17.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
List of Table
TABLE 1 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 MIDDLE EAST & AFRICA BONE MARROW DERIVED MESENCHYMAL STEM CELLS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA PLACENTAL/UMBILICAL STEM CELL IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA ADIPOSE TISSUE DERIVED MESENCHYMAL STEM CELLS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA OTHERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA MUSCULOSKELETAL DISORDERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA WOUNDS AND INJURIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA ACUTE GRAFT-VERSUS-HOST DISEASE (AGVHD) IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA SURGERIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA GASTROINTESTINAL DISEASES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA CARDIOVASCULAR DISEASES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA OTHER APPLICATION IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA HOSPITAL AND SURGICAL CENTERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA THERAPEUTIC COMPANIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA SERVICES COMPANIES IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA OTHERS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA DIRECT TENDER IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA THIRD PARTY DISTRIBUTORS IN STEM CELL THERAPY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 28 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 29 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 30 MIDDLE EAST AND AFRICA ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 31 MIDDLE EAST AND AFRICA AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 32 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 33 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 34 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 35 ISRAEL STEM CELL THERAPY MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 36 ISRAEL STEM CELL THERAPY MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 37 ISRAEL ALLOGENEIC STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 38 ISRAEL AUTOLOGOUS STEM CELL THERAPY IN STEM CELL THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 39 ISRAEL STEM CELL THERAPY MARKET, BY APPLICATIONS, 2020-2029 (USD MILLION)
TABLE 40 ISRAEL STEM CELL THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 41 ISRAEL STEM CELL THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET : SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: DBMR POSITION GRID
FIGURE 8 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: END USER COVERAGE GRID
FIGURE 10 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED INCIDENCE OF CHRONIC DISEASES, RISE IN CLINICAL TRIALS, GMP CERTIFICATION AND PRODUCT APPPROVALS IS EXPECTED TO DRIVE THE MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET FROM 2022 TO 2029
FIGURE 13 PRODUCT TYPE SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET FROM 2022 & 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET
FIGURE 15 NUMBER AND AGES OF PEOPLE 65 OR OLDER WITH ALZHEIMER'S DEMENTIA IN 2022
FIGURE 16 INCIDENCE OF VARIOUS TYPES OF CANCER IN 2020
FIGURE 17 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY PRODUCT TYPE, 2021
FIGURE 18 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY PRODUCT TYPE, 2022-2029 (USD MILLION)
FIGURE 19 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 20 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 21 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY TYPE, 2021
FIGURE 22 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 23 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 24 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY APPLICATION, 2021
FIGURE 26 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 27 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 28 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 29 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY END USER, 2021
FIGURE 30 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 31 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY END USER, CAGR (2022-2029)
FIGURE 32 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY END USER, LIFELINE CURVE
FIGURE 33 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 34 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 35 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 36 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 37 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET: SNAPSHOT (2021)
FIGURE 38 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET: BY COUNTRY (2021)
FIGURE 39 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET: BY COUNTRY (2022 & 2029)
FIGURE 40 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET: BY COUNTRY (2021 & 2029)
FIGURE 41 MIDDLE EAST AND AFRICA STEM CELL THERAPY MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 42 MIDDLE EAST & AFRICA STEM CELL THERAPY MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












